Rocking Shades in the Winter
By Frankie Wood-Black December 2018/January 2019
Teacher's Guides
Reading Supports | Tools and Resources

It’s a sunny but chilly day, and you’re ready to hit the slopes or go for a wintry walk. You’ve bundled up in warm clothes, you’ve slathered sunscreen on your face, and you may have thrown on a pair of sunglasses to reduce glare and help you see bumps and dips in the blinding white snow. Or you’ve decided to pack your goggles to prevent snow from spraying you in the eye as you swish down a mountain.
But have you ever considered that your glasses or goggles could be as important for protecting your eyes from the sun’s harsh rays as sunscreen is for protecting your skin? Your eyes are as vulnerable as your skin to the harmful effects of ultraviolet (UV) radiation. And, like your skin, your eyes need protection—whether you’re hitting the slopes or walking to school.
You can’t apply a cream or spray to your eyes, so how can you protect them? The answer is with the right pair of glasses or goggles. So, you really do have an excuse to wear shades in the winter without looking pretentious. With cold-weather fashion finally catching up to science, you have plenty of frames to choose from. The question is: What types of eyewear should you look for?
Awash with Waves
To answer that question, it’s best to understand what you’re protecting your eyes from. You’ve probably heard of UV radiation, and that too much exposure is bad for your skin. But what is UV radiation exactly?
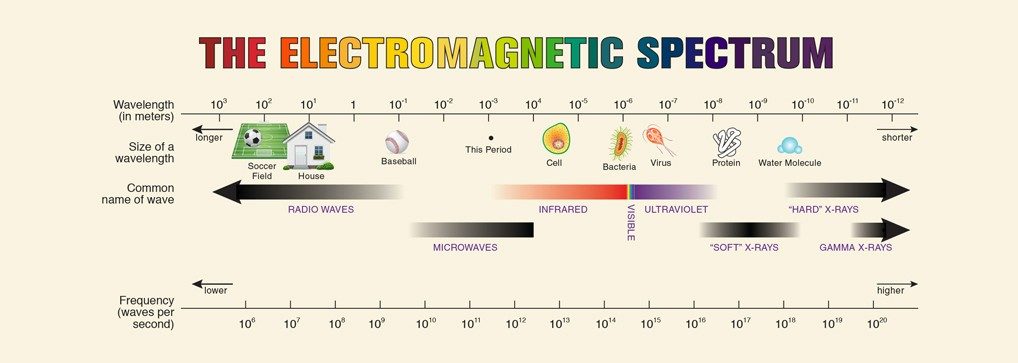
Let’s start by considering all of the radiation from the sun. The light emitted or radiated from the sun acts like both waves and particles carrying electromagnetic energy. The particles are called photons. The spectrum, or range of the waves, comprises the individual wavelengths and frequencies.
To understand these characteristics, think of ripples on the surface of a pond. The wavelength is the distance between one wave’s crest and the next. The frequency of the waves is defined as the number of complete waves or cycles that pass by a reference point during a specific time period.
Our eyes are sensitive to only a small portion of electromagnetic waves in a range that we call visible light. When all the wavelengths in the visible spectrum are mixed together, we see white light. Shine white light through a prism or view the light reflected off the back of a CD, and you’ll see the colors of the rainbow: red, orange, yellow, green, blue, indigo, and violet (a.k.a., Roy G. Biv, possibly the first art mnemonic you ever learned). This separation is called dispersion.

Dispersion occurs because each color has a different wavelength, frequency, and energy. As individual waves interact with the material of the prism or with the grooves on the CD, the light’s path bends, or refracts.
The individual colors bend differently, causing the white light to separate into varying colors. The wavelengths of visible light range from violet at 400 nanometers (nm) to red at 750 nm.
Although this portion of the electromagnetic spectrum is all humans can see, visible light waves aren’t the only waves coming from the sun (Fig. 1). The sun also emits electromagnetic radiation with wavelengths longer than 750 nm, such as infrared light, microwaves, and radio waves. It also releases radiation with wavelengths shorter than 400 nm, such as UV light from about 200 nm to 400 nm. Each wave has a corresponding energy level: the shorter the wavelength, the higher the energy. And the higher the energy, the more likely it is for those wavelengths to cause harm.
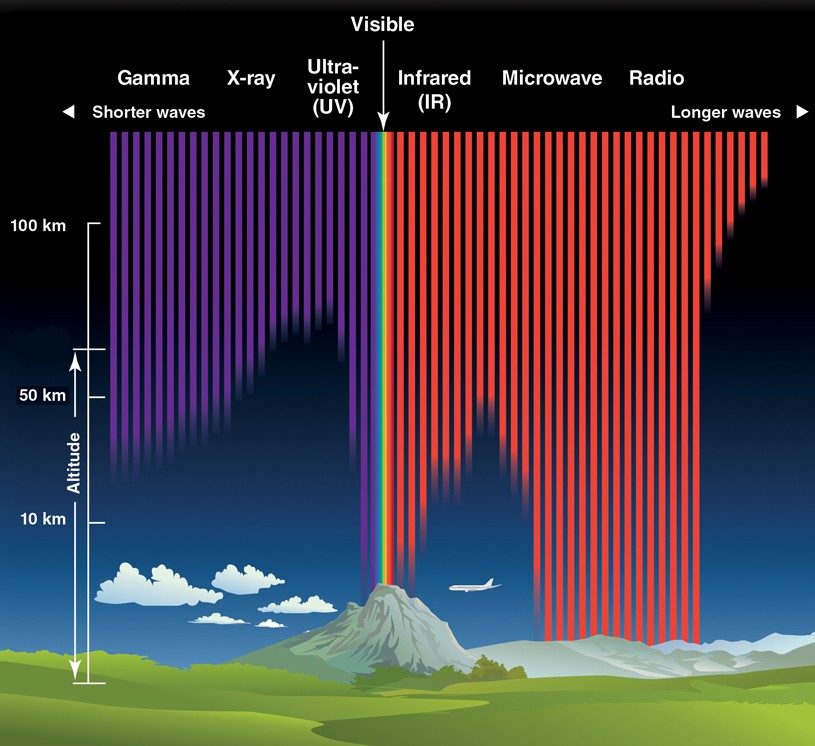
Luckily for us, Earth’s atmosphere protects us from most of the sun’s harmful rays by preventing them from reaching the surface (Fig. 2). Still, a portion of them do get through, including some UV rays. These are the ones that cause sunburn, and have the potential to cause harm to our eyes as well. Individuals who spend long hours in the sun or are exposed to highly reflective surfaces on a snowy field, for example, have a higher risk of developing cataracts, growths on the eyes, cancers, and blindness.
Choosing the right pair
So, other than staying indoors, how can you protect your eyes? Most of us put on our shades. But not all sunglasses will do the job well. To protect your eyes, you need lenses that reflect or filter out UV rays (see sidebar on the next page).
You might initially think that polarized lenses would do the trick. But you have to be careful here: These lenses filter out light based on the orientation of the rays and not on the wavelength of light.
Polarization works by filtering out some of the light waves to reduce glare—for example, from sunlight reflecting off of a road while you’re driving.
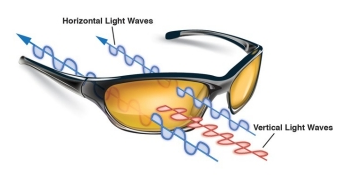
Sunglass makers can accomplish this by adding a special coating to lenses that take advantage of light’s nature. Light waves travel in various orientations—that is, in planes at different angles.
Consider just one wave. Imagine it as a wave in a string, moving up and down in a vertical plane. Think of a polarizing filter as a picket fence with gaps. If the wave in the string is traveling up and down in the same orientation as the gaps, the wave is allowed to pass through. Waves traveling at other nonvertical angles get blocked (Fig. 3). Those that bounce off of flat surfaces such as roads and the surfaces of swimming pools, for example, travel horizontally.
If you use two polarized lenses, you could orient the second lens 90 degrees from the first, and block out all light.
But polarized lenses aren’t selective for wavelength; they are only selective for the orientation of the wave. You can test your own pair by putting them on and looking at a surface with a glare, such as a car hood on a sunny day. If your lenses are polarized and you tilt your head to one side, you should notice the glare brightening.
Back to the point at hand: While polarized sunglasses reduce the amount of light you see, they will not necessarily protect your eyes from UV rays that are traveling in alignment with a polarizing filter.
To fully protect your eyes, you need lenses made with specialized materials or coatings that block UV rays. Polycarbonate is a common lens material that can do this.
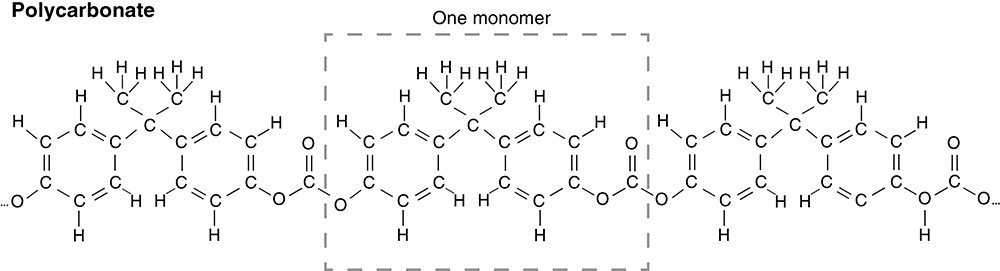
Polycarbonate is a polymer—a material where a monomer, a specific molecule, is repeated over and over to create a larger molecule (Fig. 4). Lens makers often use polycarbonate because, like glass, it is clear, but it has the added benefit of being lightweight and durable. That is, if you drop it on the floor, it probably won’t shatter.
Another reason sunglass companies like polycarbonate is that it is a natural UV filter. UV photons have the right amount of energy to excite electrons in the polycarbonate molecules to a higher energy state. The UV light thus gets absorbed by the lens and doesn’t transmit to your eye. Visible light photons aren’t energetic enough to do this, so they pass through.
Despite the availability of these materials, you still have to be careful about your eyewear choice because not all glasses and goggles are made of polycarbonate. And although polycarbonate will protect your eyes from UV light, it won’t reduce glare.
So, what you need is eyewear made with
different materials and coatings that can block UV rays, and minimize glare and brightness.
The American Academy of Ophthalmologists recommends choosing eyewear that provides 100% protection from UV rays—
the bigger, the better if you’re looking at sunglasses. Even with 100% UV protection in the lenses, rays can still enter your eyes from around the edges of glasses.
So, wear your helmet and goggles as you zip down the slopes, or a hat and sunglasses while enjoying the day. Science says: Do wear shades in the winter!
SELECTED REFERENCES
Erickson, B. E. “What’s that Stuff? Self-Darkening Eyeglasses.” Chemical & Engineering News. Vol. 87 (15), April 6, 2009: https://cen.acs.org/articles/87/i15/Self-Darkening-Eyeglasses.html.
Sousa, C.M. et al. Fast Color Change with Photochromic Fused Naphthopyrans. J. Org. Chem., Vol. 80 (24), Oct. 28, 2015: https://pubs.acs.org/doi/abs/10.1021/acs.joc.5b02116.
Buskens, P. et al. Antireflective Coatings for Glass and Transparent Polymers. Langmuir, Vol. 32 (27), May 17, 2016: https://pubs.acs.org/doi/10.1021/acs.langmuir.6b00428.
Frankie Wood-Black is a science writer based in Tonkawa, Oklahoma.
Also in this Issue...
Artificial Snow: A Slippery Slope

Are there enviornmental costs to producing artificial snow?
Searching for Patterns of Life
Searching for Patterns of Life
Ultraviolet, Ultra—Harm?
Ultraviolet light can be categorized into three types: A, B, and C. Luckily, the atmosphere blocks out UVC and much of UVB and UVA.
Without that filter, life as we know it would not exist on Earth. UVC can kill bacteria and destroy viruses. And too much UVB and UVA can potentially cause skin damage and cancer.
Still, a little UV goes a long way. Some exposure to UV light helps our bodies produce vitamin D. And scientists long ago learned how to make artificial sources of UVC to kill germs.
Here’s a breakdown of UV ray types, and their effects.
| UV Type | Wavelength | What is it? |
|---|---|---|
| UVA | 320-400 nm | The tanning rays: longest wavelengths, lowest energy |
| UVB | 280-320 nm | The sunburn rays; more energy than UVA |
| UVC | 200-280 nm | Can alter DNA: lethal to bacteria and viruses; used for sterilizing materials; highest energy UV |
Sunglasses on Demand
Polarized lenses and UV protective
sunglasses aren’t the only options for eyewear. You’ve probably also seen transition lenses, or photochromic lenses.
These lenses are made of a material that is triggered by UV light, and go from colorless to dark when you go outside.
Early photochromic lenses used silver halide crystals embedded in the lens material. But today’s version use naphthopyrans.
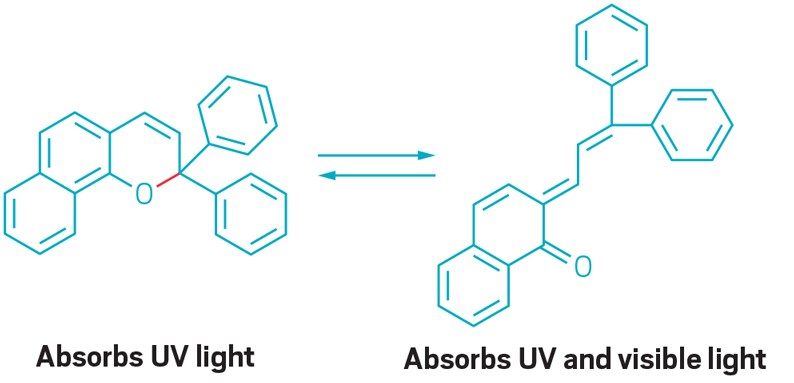
When a photon of UV light hits a naphthopyran molecule, it absorbs the energy. This absorbed energy causes the molecule’s structure to change. The new structure is able to absorb visible light photons, which cause the lens to darken. Transitions, one maker of photochromic lenses, describes the reaction as “a bit like closing the blinds in your windows on a sunny day. As the slats turn, they progressively block more and more light.”
Naphthopyrans are very sensitive to sunlight and can be synthesized easily at low cost. They can also be used in a variety of applications including glasses and windows. Another bonus of using naphthopyrans is that the reaction is reversible, allowing the molecules to return to their original shape after the energy is harmlessly released. The reverse reaction allows the lenses to become colorless again.