Mars vs. Titan: A Showdown of Human Habitability
By Kasha Patel Octoberl/November 2018
Teacher's Guides
Reading Supports | Tools and Resources

As far as we know, Earth is the only planet with life. It has accessible liquid water, food, and WiFi. But what if there is another place in the universe where humans could survive and comfortably watch Netflix? Scientists are searching for other habitable locations across the universe and are looking at two possible candidates: Mars and Saturn’s moon Titan. Studying these and other celestial bodies can help scientists learn about chemical processes that occur in our solar system and help us understand our own planet’s past, present, and future.

When thinking about living on another planet, we must first think about the conditions on Earth that help us survive. On Earth, humans can walk around on the surface, breathe oxygen, drink liquid water, survive at a comfortable temperature, and live with protection from the sun’s energetic waves. Finding all of those conditions on a different stellar object is not easy. So scientists are looking for the next best thing, with Titan and Mars in the running.
Titan’s gassy air
Titan is perhaps an unexpected candidate for human habitation because it is not even a planet, although it has many planet-like features. It is the second largest moon in our solar system, larger than our moon and the planet Mercury. More importantly, Titan is the only moon in our solar system to have an atmosphere and clouds—traits that make it similar to Earth.
Titan’s air is about 95% nitrogen (N2) and 5% methane (CH4)—coincidentally a minor ingredient in farts though not the odorous kind. It also has trace amounts of other carbon-rich compounds. The quantity of methane in Titan’s air is one of the biggest chemical differences from our home planet. Instead of water, Titan’s clouds, rain, and lakes are composed of liquid methane and ethane (C2H6).
Methane plays an important part in maintaining Titan’s thick atmosphere. It contributes to the greenhouse gas effect, which helps keep the moon’s temperatures from dropping very low. Without methane, temperatures would be low enough for nitrogen gas to condense into liquid, and the atmosphere would collapse. Here on Earth, water vapor and carbon dioxide are the predominant greenhouse gases.
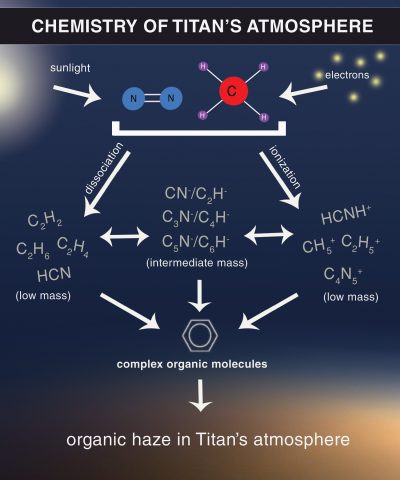
Titan’s atmospheric methane also drives the formation of more complex organic compounds. Atmospheric methane and nitrogen molecules are exposed to the sun’s ultraviolet light and high-energy particles that accelerate in Saturn’s magnetic field. This energy drives reactions with nitrogen, hydrogen, and carbon to create more complex organic compounds.
Some of those complex organic compounds are aromatic molecules, such as benzene. Others are negatively charged compounds known as carbon-chain anions (for example, CN- and C2H-). These linear compounds are thought to be the building blocks for more complex molecules and may be the basis for the earliest forms of life, including life on Earth. Scientists are not sure how or why, but these complex molecules drift lower in the atmosphere, are transformed into a complex haze of organic aerosols, and eventually reach Titan’s surface.
Life on Titan?
As it is, Titan is completely unsuitable for terrestrial life. The extremely cold temperatures of –180 °C (approximately –290 °F) would freeze any water present, and all Earth life is based on water. And while the atmospheric pressure on Titan is moderate—1.5 times the pressure of Earth’s atmosphere, about what divers experience 15 feet underwater—there is no oxygen in it. So humans would have to live in an enclosed environment that could be warmed up and filled with oxygen, perhaps produced from the frozen water that is present on Titan.
If we could manage to pull off this technological feat, would we be sharing the planet with native life forms unlike life on Earth? Scientists have speculated that perhaps life could exist that is based not on liquid water, but on liquid hydrocarbons such as methane or ethane, which are abundant on Titan. As a source of energy, such life might use the reaction of acetylene and hydrogen, two gases that are known to exist on Titan, since their combination gives off lots of energy:
C2H2 + 2 H2 fi C2H6 ∆H° = –311 kJ/mol
No one has yet seen any compelling evidence for life on Titan, and it’s hard to imagine how life would overcome the problems of operating at such low temperatures (where chemical reactions get very slow). But it would sure be cool to visit Titan to check out the possibilities!
Unfortunately, getting to Titan is tough. Unmanned satellites have taken years to reach the distant moon, and currently we do not have the technology to transport humans that far. But scientists have been exploring another possibility for our future home, and this one is much closer.
Mars: Dusty and rusty
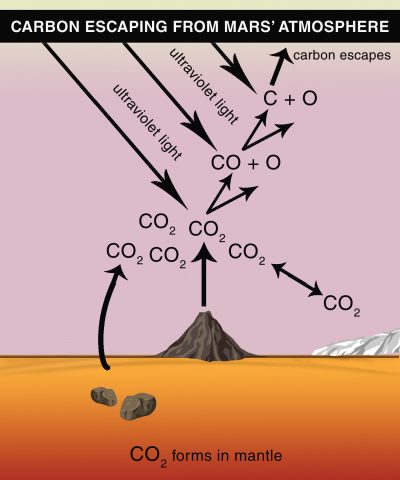
Mars is at a viable distance for human space travel—it would take about seven months to get there—but its living conditions are not ideal. The planet is blasted by harmful ultraviolet radiation. It has a weak magnetosphere that would not provide much protection from space radiation or the sun’s incoming charged particles. There is not enough oxygen for us to breathe. The Martian surface mainly features iron dust and rocks that give the planet its red, rusty color. Mars could be nicknamed “Dusty Rusty.”
So why are scientists studying ol’ Dusty Rusty? Geologic evidence on Mars suggests that the planet was once like Earth with lakes, warm weather, and possibly life. Scientists are exploring whether life could survive on present-day Mars.
One obstacle for human habitation on present-day Mars is its atmosphere. The atmosphere, primarily composed of carbon dioxide, is currently about 100 times thinner than Earth’s. The thin atmosphere makes it impossible for humans to breathe and stay warm.
How to get energy on Mars: Use iron, man
Just like on Earth and Titan, living organisms on Mars would need energy to survive. Mars has a few potential energy sources that could help microorganisms grow. The National Aeronautics and Space Administration’s (NASA) Mars rover, named Curiosity, even detected organic molecules in a rock-powder sample. But Mars does not have abundant organic material on its surface like Titan.
What it does have is a lot of iron. On Earth, microbes use energy from chemical reactions with iron in rocks—and the same could be true for Mars. Microorganisms could absorb energy from an iron reduction-oxidation reaction. The Martian environment has many electron donors and acceptors, such as iron (Fe2+, Fe3+), hydrogen (H2), perchlorate (ClO4-), and carbon monoxide (CO).
Although scientists have not yet found life on Mars, NASA is looking to send humans to Mars in the 2030s. SpaceX plans to accomplish this by 2024.
What it does have is a lot of iron. On Earth, microbes use energy from chemical reactions with iron in rocks—and the same could be true for Mars. Microorganisms could absorb energy from an iron reduction-oxidation reaction. The Martian environment has many electron donors and acceptors, such as iron (Fe2+, Fe3+), hydrogen (H2), perchlorate (ClO4-), and carbon monoxide (CO).
Although scientists have not yet found life on Mars, NASA is looking to send humans to Mars in the 2030s. SpaceX plans to accomplish this by 2024.

Survivor: Mars
Researchers have already developed a lot of the technology needed to sustain life on Mars. Spacesuits could help us withstand the low pressure, and dome bubbles could shield inhabitants from the sun’s harmful radiation and provide a breathable atmosphere. NASA is also investigating how oxygen can be obtained from the carbon-dioxide atmosphere and how to extract water from rocks. (Note: Researchers this summer reported evidence that liquid water likely exists on Mars.) Researchers have simulated Martian soil here on Earth and were able to grow simple foods, such as tomatoes and peas.
Even though Mars might be more amenable to human habitability than Titan, researchers continue to study the distant moon as they suspect the universe contains many similar celestial bodies. Thousands of other planets have been discovered in the past two decades, and many of them have conditions similar to those found on Titan. So figuring out how to potentially survive there could apply to other planets in the galaxy.
If nothing else, Titan and Mars provide insight on Earth’s past and future. Think of Earth, Titan, and Mars as a movie trilogy that tells the story of the evolution of life. Titan is the prequel showing Earth before life. Earth shows the present environment with life. Mars is the sequel to Earth, showing a post-terrestrial world. By exploring these places (and others), scientists are learning more about how life on Earth was created and what could be in store for our future.
Selected references
Gerstenmaier, W. H. Presentation to the NASA Advisory Council. Progress in Defining the Deep Space Gateway and Transport Plan, March 28, 2017.
Weber, K. A.; Achenback, L. A.; Coates, J. D. Microorganisms Pumping Iron: Anaerobic Microbial Iron Oxidation and Reduction. Nature Reviews Microbiology, Oct 1, 2006.
Schulze-Makuch D.; Grinspoon, D. H. Biologically Enhanced Energy and Carbon Cycling on Titan? Astrobiology, Aug 3, 2005. DOI: 10.1089/ast.2005.5.560.
Kasha Patel is a science writer who lives in Washington, D.C.
Also in this Issue...
The human drive to explore space

As the world continues to explore space and our place in it, some people are asking: Are the benefits of human spaceflight worth the risks?
Searching for Patterns of Life
Searching for Patterns
of Life
Searching for Patterns of Life
On Earth, we have microscopic bacteria, six-legged beetles, seven-foot tall humans, and monster-sized whales. As scientists explore the universe though, they are learning that life on another planet might look different from ours. Many planets do not have the ingredients that Earthlings need such as water. If life were to exist on another planet, the organisms might survive with a different chemistry from what we know here on Earth.
So this raises the question: How do we search for life when we do not even know its chemical composition?
It’s complicated
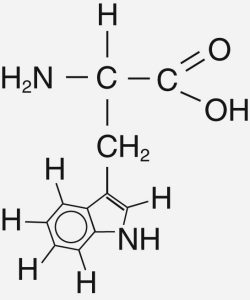
Amino acids are often called the “building blocks of life” on Earth because they make up our cells, muscles, and tissues. Scientists have found these organic molecules throughout the universe, but the most abundant types are simple structures such as glycine.
Glycine is the simplest amino acid because it has a hydrogen as its side chain, while all other amino acids have more sophisticated carbon chains.
High proportions of simple amino acids, such as glycine, on other planets show that life is rudimentary at best, or more likely, has not yet formed. Organisms might be expected to string together molecules to create more complicated amino acids, such as tryptophan, that includes an aromatic ring. Humans only use 20 amino acids, but there are many more. Even though scientists may not necessarily know which amino acids might be a sign of extraterrestrial life forms, they look for high quantities of complicated organic molecules or complex structures as a potential sign.
Time to get even
Scientists also look for even-numbered carbon chains. All known life on Earth uses carbon to live, grow, and reproduce. And the majority of our carbon chains appear in mostly even numbers because many processes in living organisms
produce carbon in pairs. In chains not produced by living organisms, carbon is added one by one, leading to both even and odd numbers of
carbons in a chain.
Measuring molecules
Researchers search for chemical compounds using spectrometers, instruments that break down the composition of an object. Like a prism, they record the light emitted from a cloud or planet and then break the signal into its individual wavelengths. Because each element or molecule gives off light in specific colors, scientists can match the colors to an individual element or molecule. Most spectrometers work with light, but some measure individual chemicals by mass.
Spectrometers are placed on many spacecraft to study the compositions of planets and other objects in space. They have helped scientists identify organic matter on Mars and complex mixtures of hydrocarbons and carbon-nitrogen compounds on Titan. With this growing body of information, scientists get closer to learning more about what kinds of organisms might exist out there—whether microscopic or monster-sized.

