The Keeling Curve: Carbon Dioxide Measurements at Mauna Loa
Dedicated at the National Oceanic and Atmospheric Administration's Mauna Loa Observatory on April 30, 2015, and Scripps Institution of Oceanography at the University of California, San Diego, on June 12, 2015.
Charles David Keeling of Scripps Institution of Oceanography was the leading authority in establishing the global atmospheric carbon dioxide (CO2) record. In 1958, Keeling began measuring atmospheric CO2 concentrations from Hawaii’s Mauna Loa Observatory. Using rigorous analytical procedures, he revealed new information about natural and man-caused carbon trends. The precision, accuracy and continuity of Keeling’s research over the span of decades provided one of the most important scientific linkages between fossil fuel combustion and global climate change due to the greenhouse effect.
Keeling’s legacy includes a measurement program that endures to this day, providing an authoritative record of atmospheric CO2 concentrations that is a cornerstone of modern climate science. The Keeling Curve, the iconic graph that presents these data, is a powerful symbol of the human impact on the environment and the role of fossil fuels in global climate change.
Contents
- Early theories and research on greenhouse gases and climate change
- International Geophysical Year, 1957–1958
- Establishing the Keeling Record at Mauna Loa
- Carbon dioxide airborne fraction
- Isotopic fingerprints and the carbon cycle
- Action on carbon dioxide emissions
- NOAA's Mauna Loa Observatory
- Biography of Charles David Keeling
- Landmark dedication and acknowledgments
- Research resources

Early theories and research on greenhouse gases and climate change
The concept of the greenhouse effect was first proposed in the 1820s by the French mathematician and physicist Joseph Fourier (1768–1830). Fourier’s calculations showed that the Earth should be much cooler than it is, given the amount of energy it receives from the sun. One explanation he proposed was that the Earth’s atmosphere might provide an insulating effect, retaining some of the heat that would otherwise be reemitted into space. Fourier’s proposal is considered the earliest hypothesis tied to the greenhouse effect.
For the next century and a half, scientists debated the connection between the composition of the atmosphere, greenhouse gas emissions (including CO2), and Earth’s temperatures. While some believed that the rapid emission of CO2 resulting from fossil fuel combustion tied to industrialization could eventually lead to a small increase in global temperatures (Svante Arrhenius, 1859–1927, first proposed this connection in 1896), others argued that natural physical processes such as the absorption of CO2 by the oceans would easily negate any increases.
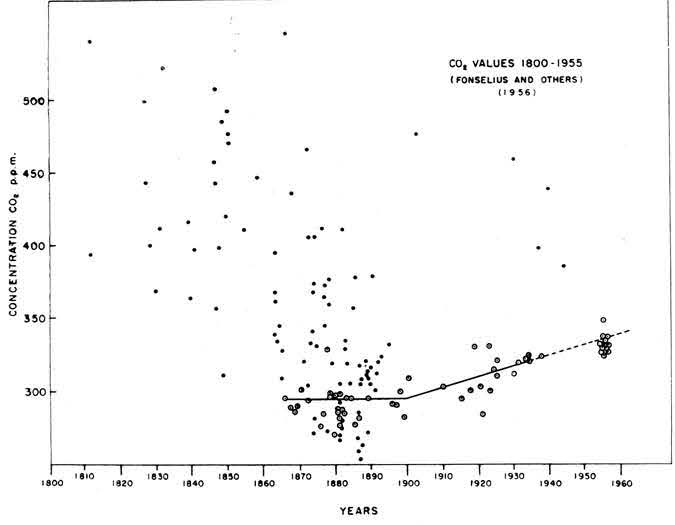
Attempts to measure atmospheric CO2 concentrations showed wide variations that were difficult to interpret. By the 1950s, what researchers needed was a precise, accurate and continuous measure of atmospheric CO2 concentrations.
At about this time Charles David Keeling (1928–2005), a postdoctoral fellow in the department of geochemistry at the California Institute of Technology (Caltech), began a research project that coupled his academic background in chemistry with his interests in geosciences and the outdoors.
Keeling began taking air and water samples every few hours throughout the day and night at remote locations in the Western states. He returned with his samples to Caltech for analysis, using a specially constructed instrument to measure CO2 amounts. The instrument, called a gas manometer, controlled for temperature, pressure and volume, down to a precision of 0.1%. Keeling was surprised to see that CO2 concentrations increased at night and decreased during the day, with a nearly constant afternoon concentration of 310 parts per million (ppm), regardless of location. These results contrasted with earlier published research that estimated much greater variability—ranging from 150 to 350 ppm.
International Geophysical Year, 1957–1958
In 1956, the U.S. Weather Bureau (now a part of the National Oceanic and Atmospheric Administration, NOAA) and other organizations in the United States and abroad were preparing research programs for the International Geophysical Year, a multinational scientific collaboration organized for the years 1957 and 1958. The Weather Bureau was planning to measure atmospheric CO2 at remote locations to establish a baseline of CO2 concentrations.
Keeling presented his findings to Harry Wexler (1911–1962), director of the Weather Bureau’s Division of Meteorological Research. Wexler was impressed by Keeling’s methods and offered him the job of leading the Weather Bureau’s proposed CO2 program. Shortly after, Keeling received an offer from Roger Revelle (1909–1991), director of Scripps Institution of Oceanography, to conduct his Weather Bureau research from the Scripps campus in La Jolla, California.
Keeling had proposed to both Wexler and Revelle that he could deploy a new analytical tool called an infrared gas analyzer to perform continuous measurements of CO2 in air samples. The analyzers, designed by the Applied Physics Corporation, would be calibrated using the manometric technique that Keeling had employed earlier in his career to obtain highly precise data. The Weather Bureau offered to support analyzers at locations in Hawaii and Antarctica, and a third aboard a research ship. Additional samples would be collected in glass flasks at remote locations and onboard research aircraft and returned to Scripps for analysis.
Keeling moved to La Jolla in August of 1956. He immediately began the difficult task of preparing the equipment and protocol and hiring staff needed to implement the CO2 program, which was scheduled to begin the following July.
The first analyzer was sent to the Little America station in Antarctica in late 1956, but equipment problems rendered it unusable until the following year. The first of the remote flask samples was collected at the South Pole in early 1957 and sent back to Scripps for analysis, providing the earliest data from the program. A second analyzer was prepared for installation aboard a Scripps research ship, set to launch in the fall of 1957. Sampling by U.S. Air Force aircraft was the next priority.

Establishing the Keeling Record at Mauna Loa
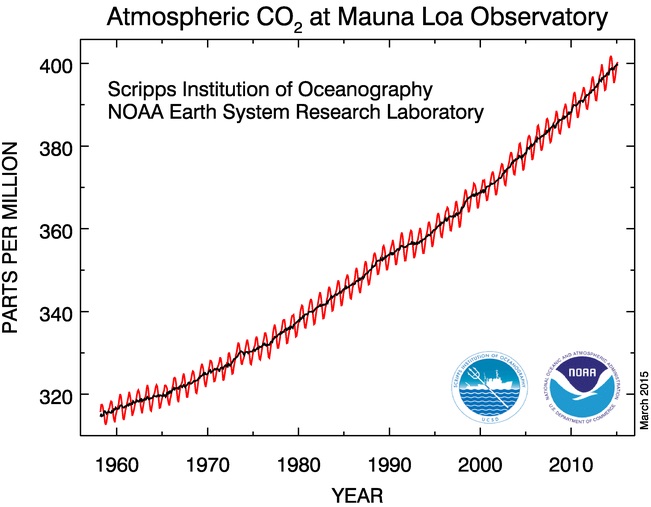
In March 1958, an analyzer was installed at the Weather Bureau’s Mauna Loa Observatory. The observatory is located on the remote north slope of the Mauna Loa volcano, one of several volcanoes on the Island of Hawaii (commonly known as the Big Island), and it is an ideal location for collecting pristine air far from human influences. Weather Bureau employees were responsible for the difficult work of obtaining measurements and maintaining the instrument, and they relayed data to Keeling in California for further analysis. The first reading from Mauna Loa, dated March 29, 1958, measured the atmospheric CO2 concentration at 313 ppm.
Daily averages were recorded until power failures interrupted the equipment between May and July. These data from the first three months showed a progressive increase in CO2 concentrations. When testing resumed in July, a decrease was registered. Additional equipment failures resulted in lapses in the records for September and October. By November, CO2 levels showed a new low, only to increase in the following months. In 1978, Keeling recalled, “I became anxious that the concentration was going to be hopelessly erratic.”
When a full year of measurements was completed in 1959, a pattern emerged that began to make sense. The appearance of seasonal oscillations of CO2, with peaks in May and lows in November, reflected the impact of vegetation cycles that prevail across the northern hemisphere: Plants take in CO2 during the growing period lasting from April through August in a process called photosynthesis, thus reducing atmospheric CO2 levels during these months. In the winter when plants lose their foliage, carbon stored within plant tissues and soils is released to the atmosphere, increasing CO2 concentrations.
Keeling reported his initial findings in the geophysics journal Tellus in 1960, describing the seasonal pattern of CO2 variations. The pattern, never before observed, is now known as Earth’s breathing cycle. Near the end of his report, Keeling pointed out that “[w]here data extend beyond one year, averages for the second year are higher than for the first year.” He went on to say that Antarctic data suggested that the increase could be associated with fossil fuel combustion. Although Keeling refrained at this time from drawing conclusions about the source of the increasing portion of CO2, it is clear that he recognized the role that CO2 measurements could play in understanding the linkage between fossil fuel emissions and atmospheric change.
Although Revelle intended that the CO2 measurements taken during the International Geophysical Year would obtain a baseline measurement, to be repeated again after a decade or longer for comparison, Keeling’s data offered results that led to the program becoming a long-term study. Given what Keeling and other scientists knew about the effect of CO2 as a greenhouse gas, there were strong reasons to continue the monitoring program, uninterrupted.


High-resolution infographic
Carbon dioxide airborne fraction
As Keeling amassed actual measurements over a series of years, the yearly increase in atmospheric CO2 became more apparent. For each succeeding year of the record, the average annual CO2 level rose. A clear picture of how fossil fuel emissions, the greenhouse effect and climate change are connected began to come into focus.
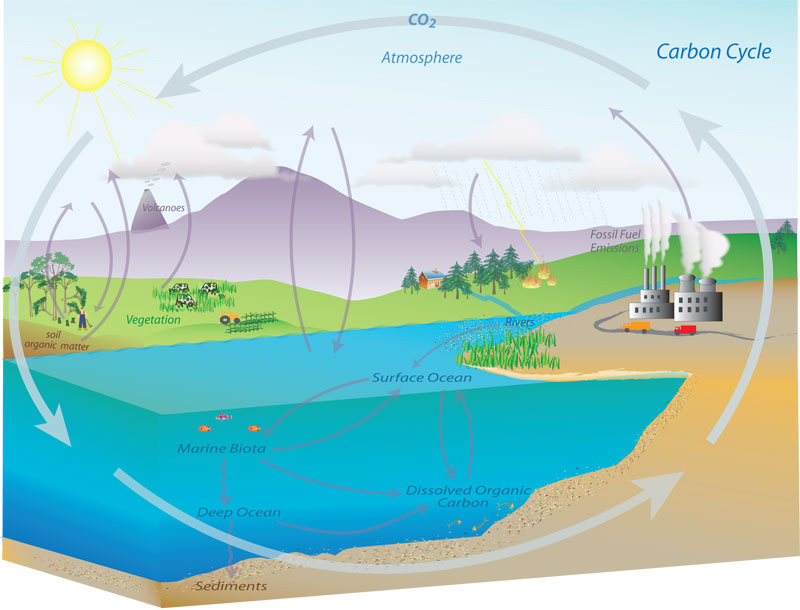
Keeling’s data also shed new light on the carbon cycle, in which carbon moves between Earth’s oceans, atmosphere, biosphere and geosphere. As humans exploited fossil fuels such as coal, oil and natural gas (starting in large quantities during the Industrial Revolution), they released the carbon stored therein into the atmosphere as CO2.
While scientists had long theorized that increases in CO2 emissions released by fossil fuel combustion might lead to increased global temperatures as a result of the greenhouse effect, this idea had remained largely unproven. Keeling’s data finally provided real atmospheric measurements—not estimates—that scientists could use to further their understanding of these connections.
Using these data, Keeling was able to compare the amount of CO2 accumulating in the atmosphere against estimates of the amount of CO2 being released by burning fossil fuels. The atmospheric fraction appeared to be approximately 55%, meaning that roughly half of all CO2 released by coal, oil and natural gas was remaining in the atmosphere, thus causing the Keeling Curve’s annual rise. This figure, reported by Keeling and colleagues at Scripps in 1973, is known as the “airborne fraction.” The remainder is dissolved into the oceans, taken up by plants or accumulated in soils.
Knowing the airborne fraction is necessary not only for scientists who look at the present and past to describe the impacts humans have had on the atmosphere, but also for those who look ahead. The observed airborne fraction is critical in developing models that project future climate effects of rising greenhouse gas concentrations—effects that include rising temperatures and sea levels, ocean acidification and other phenomena.
Keeling’s research disproved widely-held beliefs in the scientific community that exchanges within the carbon cycle could mitigate rising CO2 levels. And the continued annual increase in CO2 concentrations, accelerating over the decades, revealed the still-growing impact of fossil fuel combustion.
Isotopic fingerprints and the carbon cycle
In the late 1970s, Keeling’s group undertook a long-planned expansion of the CO2 program that looked more closely at sources of the CO2 that are present in the atmosphere.
Carbon can be distinguished by its isotopes—carbon atoms with varying numbers of neutrons. Carbon atoms containing six protons and six neutrons (known as carbon-12 or 12C) are the most common isotope and comprise about 99% of all carbon on Earth. Those that contain an additional neutron are carbon-13, 13C, which comprise about 1% of all carbon. Carbon-14, 14C, with two extra neutrons, makes up only a trace amount. The three isotopes behave very similarly in chemical reactions, so they move through the environment in nearly identical ways.
The isotopes 12C and 13C are stable, meaning they are conserved over time, while 14C is unique among the three for being unstable. This isotope, also called radiocarbon, is created in nature when cosmic rays act upon nitrogen atoms in the atmosphere (atomic tests have also contributed some 14C). It has a constant, well-known half-life, meaning that 14C disappears at a predictable rate by turning back into nitrogen.
Because of that regular disintegration, radiocarbon can be used to determine the age of carbon-based objects like fossilized plants and animals. The less 14C an object has, the older it must be. In contrast, the amount of 12C and 13C remains unchanged after many thousands of years. Therefore, modern sources of CO2 such as living organisms have about the same amount of 14C as the atmosphere, whereas ancient sources like fossil fuels have none left.
Scientists use analytical tools to determine the signature ratios of 12C, 13C and 14C found in different reservoirs (the oceans, atmosphere, biosphere, fossil fuels and others). These ratios are sometimes called carbon fingerprints. Using these fingerprints, scientists can compare the ratios of carbon isotopes over time to track carbon as it moves from one reservoir to the next. For example, as fossil fuels are burned, they add 12C and 13C to the atmosphere, but no 14C. The result is that the proportion of 14C in the atmosphere goes down.
Keeling had made isotopic measurements previously while conducting experiments in the woodlands of California, and he had planned to continue isotopic measurements of his air samples as part of the International Geophysical Year program. Difficulties in analysis at that time led him to sideline this research. In 1978, Keeling returned to the isotopic studies, working with a group at the University of Groningen in the Netherlands to study the isotopic ratios of carbon present in samples dating back to 1955.
The first paper on isotopic measurements from this period was published in 1979. It showed a shift in 13C/12C ratio that matched predictions associated with fossil fuel combustion. Further analysis of 14C was used as evidence that the current accumulation of CO2 in the atmosphere was linked to the liberation of long-sequestered banks of carbon—the burning of fossil fuels by mankind.
Action on carbon dioxide emissions
By the 1960s, a wide array of human-caused environmental issues had become a serious concern among scientists and the public, including greenhouse gas emissions and their link to global climate change.
In 1965, President Lyndon B. Johnson’s Science Advisory Committee addressed pollution issues in the country’s air, water and land. Revelle, then at Harvard University, served on the committee’s Environmental Pollution Panel and chaired a working group on atmospheric CO2 whose membership included Keeling. The working group contributed the portion of the report that focused on CO2, or the “invisible pollutant,” as the report identified it.
A subsection of the report explored possible effects of increased atmospheric CO2 on Earth’s climate. These ranged from significant increases in average global temperatures, to melting of arctic ice sheets and the resulting rise in sea levels, to increased acidity of water bodies. The report concluded: “Through his worldwide industrial civilization, Man is unwittingly conducting a vast geophysical experiment.”
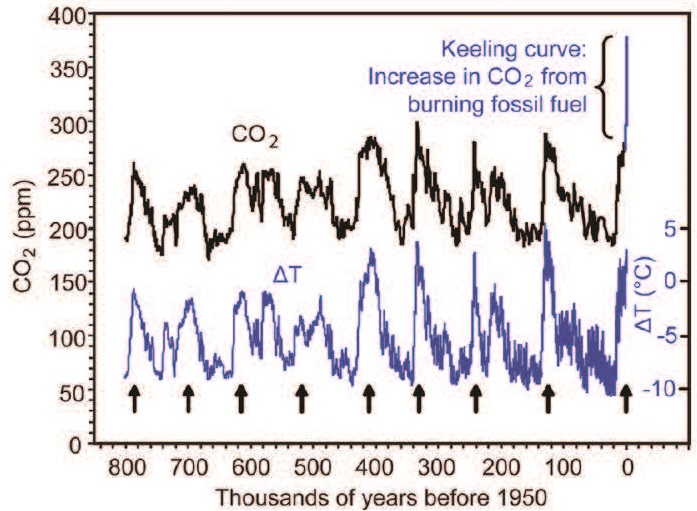
Research on air samples trapped within polar ice deposits, conducted in the 1980s, revealed levels of CO2 dating back hundreds of thousands of years. These data extended the Keeling Curve and showed a startling surge in CO2 concentrations to levels not seen in more than 800,000 years. With the pace of utilization of fossil fuels rapidly increasing, so too were CO2 levels, reaching heights that clearly exceed natural trends.
Attention to greenhouse gases and their role in climate change intensified, and in 1988 the United Nations created the Intergovernmental Panel on Climate Change (IPCC) to prepare reports and recommendations about climate change based on scientific information. Its first assessment, issued in 1990, outlined the importance of international coordination on such issues and set the stage for subsequent United Nations actions to mitigate climate change by addressing its cause—greenhouse gases contributed by human activity.
In 1992, the United Nations Framework Convention on Climate Change was ratified, with the objective to “stabilize greenhouse gas concentrations in the atmosphere at a level that would prevent dangerous anthropogenic [man-made] interference with the climate system.” Five years later, the Kyoto Protocol emerged, the first international agreement that committed member countries to reductions in CO2 and other greenhouse gases.
Nearly 40 years after Keeling’s data first showed an increase in CO2 in the atmosphere—evidence that supported theories connecting greenhouse gas emissions and climate change which dated back to the 1800s—significant action to reduce emissions was finally enacted.
NOAA's Mauna Loa Observatory
Although prior scientific expeditions and installations had taken place in the area, today’s Mauna Loa Observatory (MLO) was dedicated on June 28, 1956, as the first permanent research settlement atop the Mauna Loa volcano on the Island of Hawaii. Originally established by the U.S. Weather Bureau, the observatory is now a part of the Global Monitoring Division within the National Oceanic and Atmospheric Administration. It has been the site of important atmospheric, solar and other geosciences research for more than 50 years.
MLO sits at an elevation of 11,135 feet, near the summit of Hawaii’s second tallest mountain. An active volcano, Mauna Loa occasionally emits CO2, sulfur dioxide and other gases that can disrupt the open-air data being recorded but which are easily detectable. Despite this and other challenges relating to its remote location, MLO provides researchers with a nearly pristine site for atmospheric and solar observations, due to its setting far from human activity, vegetation and dust. A strong inversion layer further suppresses local pollutants from lower elevations.
Since 1958, atmospheric CO2 concentrations have been analyzed at Mauna Loa nearly continuously in a research program based at Scripps Institution of Oceanography. NOAA began its own measurements of CO2 concentrations at Mauna Loa in 1974. The two programs have been maintained in parallel for more than 40 years, together making the Mauna Loa data the world’s foremost continuous record of CO2 concentrations in the atmosphere.

Biography of Charles David Keeling
Charles David Keeling’s career in CO2 monitoring resulted from the combination of his academic background in science and his interest in the outdoors. Applying his Ph.D. in chemistry from Northwestern University (1954) with his love of natural places in the western U.S., Keeling joined the new geochemistry department at the California Institute of Technology in Pasadena, California. Within a few years, Keeling had undertaken a research program built on precise sampling of atmospheric CO2 in Big Sur, California, and other wilderness areas. This research left him perfectly positioned to lead the U.S. Weather Bureau’s CO2 monitoring program during the International Geophysical Year, which stretched from July 1957 to December 1958.
Over a more than 50-year career, Keeling earned renown as the world’s foremost specialist in atmospheric CO2 studies through his persistence at developing a continuous and precise record of the CO2 concentrations in the Earth’s atmosphere.
Keeling was affiliated with Scripps Institution of Oceanography from 1956 until his death in 2005. He published nearly 100 research articles on atmospheric chemistry and the carbon cycle. Keeling faced the possible or even likely demise of his program numerous times, but at each point he persisted in his research. During his career he developed connections between his data and climate change that would alter the way geosciences were studied and influence popular opinion as well as governmental policies regarding the use of fossil fuels. The Mauna Loa program was a high-profile example of the importance of long-term research to advancing scientific understanding of Earth phenomena.
Keeling received numerous accolades during his career. He was elected a fellow of the American Academy of Arts and Sciences in 1986 and a member of the National Academy of Sciences in 1994. In 2002 Keeling was awarded the National Medal of Science, the nation’s highest award for lifetime achievements in science. In 2005, he received the Tyler Prize for Environmental Achievement for his data collection and interpretation.
Landmark dedication and acknowledgments
Landmark dedication
The American Chemical Society dedicated the Keeling Curve as a National Historic Chemical Landmark in ceremonies at NOAA's Mauna Loa Observatory in Hilo, Hawaii, on April 30, 2015, and at Scripps Institution of Oceanography at the University of California, San Diego, on June 12, 2015.
The commemorative plaque at Mauna Loa Observatory reads
In 1958, Charles David Keeling (1928–2005) of Scripps Institution of Oceanography began a cooperative program for the study of atmospheric carbon dioxide (CO2) at the newly established Mauna Loa Observatory of the U.S. Weather Bureau (now a part of NOAA). By 1960, Keeling revealed two significant findings, reporting the first quantitative estimate of Earth’s natural seasonal CO2 oscillations while also discovering a steady annual increase in CO2, the most significant greenhouse gas contributing to global climate change. Keeling advanced our understanding of mankind’s impact on Earth by linking fossil fuel emissions to rising levels of CO2. His dedication to continuous and accurate measurements enabled these data to become an unequivocal record of the global rise in CO2 and an icon of atmospheric science.
The commemorative plaque at Scripps Institution of Oceanography reads
In 1958, Charles David Keeling (1928–2005) of Scripps Institution of Oceanography began a cooperative program for the study of atmospheric carbon dioxide (CO2) at the newly established Mauna Loa Observatory of the U.S. Weather Bureau and other sites around the world. By 1960, Keeling revealed two significant findings, reporting the first quantitative estimate of Earth’s natural seasonal CO2 oscillations while also discovering a steady annual increase in CO2, the most significant greenhouse gas contributing to global climate change. Keeling advanced our understanding of mankind’s impact on Earth by linking fossil fuel emissions to rising levels of CO2. His dedication to continuous and accurate measurements enabled these data to become an unequivocal record of the global rise in CO2 and an icon of atmospheric science.
Acknowledgments
Adapted for the internet from “The Keeling Curve,” produced by the National Historic Chemical Landmarks program of the American Chemical Society in 2015.


Research resources
Further reading
- The Keeling Curve (National Historic Chemical Landmark booklet; PDF)
- Scripps CO2 Program (Scripps Institution of Oceanography, UC San Diego)
- Mauna Loa Observatory (National Oceanographic and Atmospheric Administration)
- Education and Ourtreach (NOAA Earth System Research Laboratory, Global Monitoring Division)
Cite this page
American Chemical Society National Historic Chemical Landmarks. The Keeling Curve. http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/keeling-curve.html (accessed Month Day, Year).



