Willard Libby and Radiocarbon Dating
Dedicated at the University of Chicago on October 10, 2016.
In 1946, Willard Libby proposed an innovative method for dating organic materials by measuring their content of carbon-14, a newly discovered radioactive isotope of carbon. Known as radiocarbon dating, this method provides objective age estimates for carbon-based objects that originated from living organisms. The “radiocarbon revolution” made possible by Libby’s discovery greatly benefitted the fields of archaeology and geology by allowing practitioners to develop more precise historical chronologies across geography and cultures.
Willard Libby's concept of radiocarbon dating
Willard Libby (1908–1980), a professor of chemistry at the University of Chicago, began the research that led him to radiocarbon dating in 1945. He was inspired by physicist Serge Korff (1906–1989) of New York University, who in 1939 discovered that neutrons were produced during the bombardment of the atmosphere by cosmic rays. Korff predicted that the reaction between these neutrons and nitrogen-14, which predominates in the atmosphere, would produce carbon-14, also called radiocarbon.
Libby cleverly realized that carbon-14 in the atmosphere would find its way into living matter, which would thus be tagged with the radioactive isotope. Theoretically, if one could detect the amount of carbon-14 in an object, one could establish that object’s age using the half-life, or rate of decay, of the isotope. In 1946, Libby proposed this groundbreaking idea in the journal Physical Review.
You read statements in books that such and such a society or archeological site is 20,000 years old. We learned rather abruptly that these numbers, these ancient ages, are not known accurately; in fact, it is at about the time of the First Dynasty in Egypt that the first historical date of any real certainty has been established.”
—Willard Libby, Nobel Lecture, 12 December 1960
Predictions about carbon-14
The concept of radiocarbon dating focused on measuring the carbon content of discreet organic objects, but in order to prove the idea Libby would have to understand the earth’s carbon system. Radiocarbon dating would be most successful if two important factors were true: that the concentration of carbon-14 in the atmosphere had been constant for thousands of years, and that carbon-14 moved readily through the atmosphere, biosphere, oceans and other reservoirs—in a process known as the carbon cycle.
In the absence of any historical data concerning the intensity of cosmic radiation, Libby simply assumed that it had been constant. He reasoned that a state of equilibrium must exist wherein the rate of carbon-14 production was equal to its rate of decay, dating back millennia. (Fortunately for him, this was later proven to be generally true.)
For the second factor, it would be necessary to estimate the overall amount carbon-14 and compare this against all other isotopes of carbon. Based on Korff’s estimation that just two neutrons were produced per second per square centimeter of earth’s surface, each forming a carbon-14 atom, Libby calculated a ratio of just one carbon-14 atom per every 1012 carbon atoms on earth.
Libby’s next task was to study the movement of carbon through the carbon cycle. In a system where carbon-14 is readily exchanged throughout the cycle, the ratio of carbon-14 to other carbon isotopes should be the same in a living organism as in the atmosphere. However, the rates of movement of carbon throughout the cycle were not then known. Libby and graduate student Ernest Anderson (1920–2013) calculated the mixing of carbon across these different reservoirs, particularly in the oceans, which constitute the largest reservoir. Their results predicted the distribution of carbon-14 across features of the carbon cycle and gave Libby encouragement that radiocarbon dating would be successful.
The Keeling Curve
The carbon cycle features prominently in the story of chemist Ralph Keeling, who discovered the steadily increasing carbon dioxide concentrations of the atmosphere. Learn more.
Detecting radiocarbon in nature
Carbon-14 was first discovered in 1940 by Martin Kamen (1913–2002) and Samuel Ruben (1913–1943), who created it artificially using a cyclotron accelerator at the University of California Radiation Laboratory in Berkeley. Further research by Libby and others established its half-life as 5,568 years (later revised to 5,730 ± 40 years), providing another essential factor in Libby’s concept. But no one had yet detected carbon-14 in nature— at this point, Korff and Libby’s predictions about radiocarbon were entirely theoretical. In order to prove his concept of radiocarbon dating, Libby needed to confirm the existence of natural carbon-14, a major challenge given the tools then available.
At the time, no radiation-detecting instrument (such as a Geiger counter) was sensitive enough to detect the small amount of carbon-14 that Libby’s experiments required. Libby reached out to Aristid von Grosse (1905–1985) of the Houdry Process Corporation who was able to provide a methane sample that had been enriched in carbon-14 and which could be detected by existing tools. Using this sample and an ordinary Geiger counter, Libby and Anderson established the existence of naturally occurring carbon-14, matching the concentration predicted by Korff.
This method worked, but it was slow and costly. Fortunately, Libby’s group developed an alternative. They surrounded the sample chamber with a system of Geiger counters that were calibrated to detect and eliminate the background radiation that exists throughout the environment. The assembly was called an “anti-coincidence counter.” When it was combined with a thick shield that further reduced background radiation and a novel method for reducing samples to pure carbon for testing, the system proved to be suitably sensitive. Finally, Libby had a method to put his concept into practice.
Testing radiocarbon dating
The concept of radiocarbon dating relied on the ready assumption that once an organism died, it would be cut off from the carbon cycle, thus creating a time-capsule with a steadily diminishing carbon-14 count. Living organisms from today would have the same amount of carbon-14 as the atmosphere, whereas extremely ancient sources that were once alive, such as coal beds or petroleum, would have none left. For organic objects of intermediate ages—between a few centuries and several millennia—an age could be estimated by measuring the amount of carbon-14 present in the sample and comparing this against the known half-life of carbon-14.
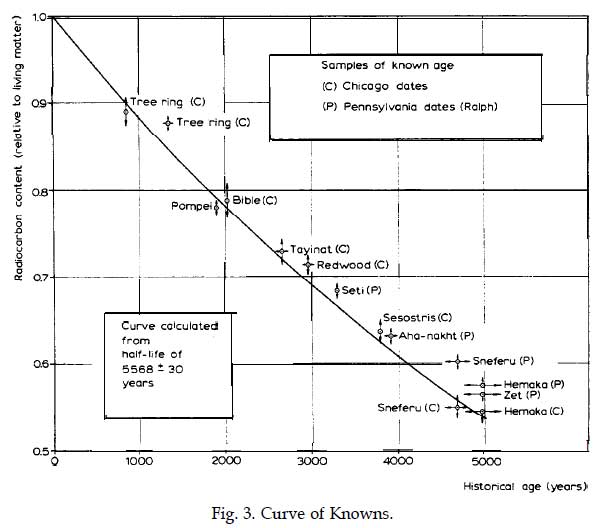
To test the technique, Libby’s group applied the anti-coincidence counter to samples whose ages were already known. Among the first objects tested were samples of redwood and fir trees, the age of which were known by counting their annual growth rings. They also sampled artifacts from museums such as a piece of timber from Egyptian pharaoh Senusret III’s funerary boat, an object whose age was known by the record of its owner’s death.
In 1949, Libby and Arnold published their findings in the journal Science, introducing the “Curve of Knowns.” This graph compared the known age of artifacts with the estimated age as determined by the radiocarbon dating method. It showed all of Libby’s results lying within a narrow statistical range of the known ages, thus proving the success of radiocarbon dating.
The "Radiocarbon Revolution"
The introduction of radiocarbon dating had an enormous influence on both archaeology and geology—an impact often referred to as the “radiocarbon revolution.” Before Libby’s research, investigators in these fields had to rely on methods of dating that were merely relative, such as comparing the layers of a site in which artifacts were found, presuming that the layers of a site were laid down chronologically. Relative dating simply places events in order without a precise numerical measure. By contrast, radiocarbon dating provided the first objective dating method—the ability to attach approximate numerical dates to organic remains.
This method helped to disprove several previously held beliefs, including the notion that civilization originated in Europe and diffused throughout the world. By dating man-made artifacts from Europe, the Americas, Asia, Africa and Oceania, archaeologists established that civilizations developed in many independent sites across the world. As they spent less time trying to determine artifact ages, archaeologists were able to ask more searching questions about the evolution of human behavior in prehistoric times.
Libby’s work also contributed greatly to geology. By using wood samples from trees once buried under glacial ice, Libby proved that the last ice sheet in northern North America receded 10,000 to 12,000 years ago, not 25,000 years as geologists had previously estimated.
When Libby first presented radiocarbon dating to the public, he humbly estimated that the method may have been able to measure ages up to 20,000 years. With subsequent advances in the technology of carbon-14 detection, the method can now reliably date materials as old as 50,000 years.
Seldom has a single discovery in chemistry had such an impact on the thinking in so many fields of human endeavor. Seldom has a single discovery generated such wide public interest.”
—Kenneth Pitzer, Nobel Prize in Chemistry nomination for Willard Libby
Biography of Willard Libby
Willard Frank Libby was born in Grand Valley, Colorado, on Dec. 17, 1908. He studied chemistry at the University of California, Berkeley, receiving a bachelor’s degree in 1931 and a Ph.D. in 1933. In 1941, Libby was awarded a Guggenheim Fellowship, but his plans were interrupted by the United States’ entry into World War II. He went to Columbia University instead, working to produce enriched uranium for the nation’s atomic weapons program.
When the war ended, Libby became a professor in the Department of Chemistry and Institute for Nuclear Studies (now The Enrico Fermi Institute) of the University of Chicago. It was here that he developed his theory and method of radiocarbon dating, for which he was awarded the Nobel Prize in Chemistry in 1960.
Libby left Chicago in 1954 upon his appointment as a commissioner of the U.S. Atomic Energy Commission. In 1959, Libby returned to teaching at the University of California, Los Angeles, where he remained until his retirement in 1976. Libby died in 1980 at the age of 71.
Landmark dedication and acknowledgments
Landmark dedication
The American Chemical Society designated the discovery of radiocarbon dating as a National Historic Chemical Landmark at the University of Chicago in Chicago, Illinois, on October 10, 2016. The commemorative plaque reads:
In 1946, Willard Libby (1908–1980) developed a method for dating organic materials by measuring their content of carbon-14, a radioactive isotope of carbon. The method is now used routinely throughout archaeology, geology and other sciences to determine the age of ancient carbon-based objects that originated from living organisms. Libby’s discovery of radiocarbon dating provides objective estimates of artifact ages, in contrast to previous methods that relied on comparisons with other objects from the same location or culture. This “radiocarbon revolution” has made it possible to develop more precise historical chronologies across geography and cultures. For this discovery, Libby received the Nobel Prize in Chemistry in 1960.
Acknowledgments
Adapted for the internet from "Discovery of Radiocarbon Dating," produced by the American Chemical Society's National Historic Chemical Landmarks program in 2016.
Research resources
Further reading
- Discovery of Radiocarbon Dating (American Chemical Society NHCL booklet; PDF)
- The Nobel Prize in Chemistry, 1960 (NobePrize.org)
- CHF acquires instrument that played a role in the development of carbon-14 dating (Chemical Heritage Foundation)
- Carbon-14 is 75±0 Years Old (Smithsonian National Museum of American History)
Cite this page
American Chemical Society National Historic Chemical Landmarks. Discovery of Radiocarbon Dating. http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/radiocarbon-dating.html (accessed Month Day, Year).