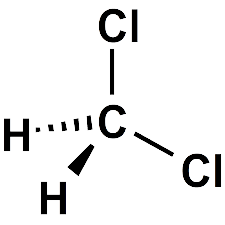
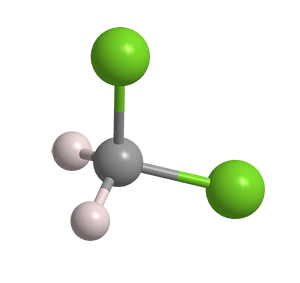
What molecule am I?
March is MOTW Solvent Month! This is the first of four articles about key solvents — Ed.
Dichloromethane, commonly called methylene chloride, is a solvent that is widely used in chemical research and manufacturing. It is a highly volatile liquid (see fast facts table), but it is neither flammable nor explosive in air.
Dichloromethane is commonly produced by chlorinating methane. The process also produces the other three C1 chlorohydrocarbons—chloromethane, trichloromethane (chloroform), and tetrachloromethane (carbon tetrachloride). The four are separated via distillation.
Although dichloromethane is the least toxic C1 chlorohydrocarbon, it does present hazards. Inhaling it can produce symptoms ranging from drowsiness to respiratory tract irritation and even death. It also may be carcinogenic, but not enough studies have been done to establish the degree of exposure that causes cancer.
Despite its health risks, dichloromethane is one of the main solvents used to decaffeinate coffee beans. After the caffeine is removed, the solvent’s volatility makes it easy to remove residual solvent. Any remaining dichloromethane is well below the 10-ppm concentration allowed by the US Food and Drug Administration.
Dichloromethane hazard information
| GHS classification*: skin irritation, category 2 | |
| H315—Causes skin irritation | |
| GHS classification: serious eye irritation, category 2A | |
| H319—Causes serious eye irritation | |
| GHS classification: specific target organ toxicity, single exposure, narcotic effects, category 3 | |
| H336—May cause drowsiness or dizziness | |
| GHS classification: carcinogenicity, category 2 | |
| H351—Suspected of causing cancer | |
| GHS classification: hazardous to the aquatic environment, acute hazard, category 3 | |
| H402—Harmful to aquatic life | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW update: April 01, 2019
Dichloromethane (aka methylene chloride) has been a useful sovlent for decades; but its significant hazards have caused it to be used in a declining number of applications. In mid-March, the US Environmental Protection Agency finalized a ban on dichloromethane as an ingredient in paint and coating removers used by consumers, effective at the end of 2019. Environmental and health activists say that the ban, originally promulgated during the Obama administration for consumer and industrial use, does not go far enough.
Molecule in the News
MOTW highlights molecules that appear in major news outlets. See this week's edition.
Dichloromethane fast facts
| CAS Reg. No. | 75-09-2 |
| Empirical formula | CH2Cl2 |
| Molar mass | 84.93 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 40 ºC |
| Water solubility | 13 g/L |
MOTW update:
November 18, 2019
Former Molecules of the Week 1,4-dioxane, hexabromocyclododecane, 1-bromopropane, N-methyl-2-pyrrolidone, and dichloromethane, among several other chemicals, are at the center of a controversy about how the EPA’s scientific advisory committees are evaluating risks of air pollution. The problems are the lack of expertise in some of the committees and a shortage of adquate data to evaluate the chemicals. Industrial and environmental stakeholders are critical of the latest risk assessments.
Molecule in the News
Dichlromethane1 (methylene chloride, CH2Cl2), the Molecule of the Week for March 4, 2019, is a widely used solvent that has come under increasing pressure from health and environmental regulatory agencies in the past several years.
In the latest move this April, the US Environmental Protection Agency proposed a ban on dichloromethane for all consumer and most industrial and commercial uses, including adhesives, paint strippers, and degreasers. Dichloromethane is the second chemical to be reviewed under the 2016 revision of the Toxic Substances Control Act. The first was asbestos2, and the third is expected to be tetrachloroethylene3.
1. CAS Reg. No. 75-09-2.
2. CAS Reg. No. 1332-21-4.
3. CAS Reg. No. 127-18-4.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.


