What molecule am I?
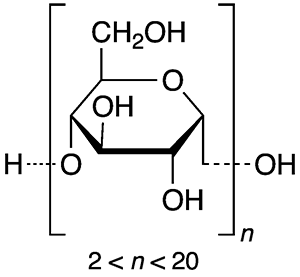
Maltodextrin is a polysaccharide that is used primarily in foods and beverages as a thickener, sweetener, and/or stabilizer. It is a relatively short-chain polymer (some would call it an oligomer); commercial products contain an average of ≈3 to ≈17 glucose units per chain. It is manufactured by partially hydrolyzing grain starches, usually corn or wheat.
Because maltodextrin is safe, inexpensive, and extremely water-soluble, it is used widely as a food additive in a variety of products, ranging from infant formula to ice cream to salad dressing to peanut butter to beer. It is a supplemental ingredient in sweeteners such as sucralose and stevia.
Maltodextrin is not as good a sweetening agent as sucrose (common sugar), but it has as much calorie content as the equivalent amount of sugar. Obese individuals and diabetics should be aware that a food contains maltodextrin before consuming it; it is a listed ingredient on food labels.
Despite these caveats, maltodextrin consumption is increasing steadily. According to Business Wire, the market will expand by >5% annually through 2020, when global sales will reach >US$3 billion.
Maltodextrin hazard information
GHS classification*: not a hazardous substance or mixture |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Maltodextrin fast facts
| CAS Reg. No. | 9050-36-6 |
| Molar mass | 504.5 g/mol (n = 3) 2774.7 g/mol (n = 17) |
| Empirical formula | C6nH(10n+2)O(5n+1) |
| Appearance | White to yellow powder |
| Melting point | 240 ºC (dec.) |
| Water solubility | ≈1.2 kg/L |
*n = number of glucose units.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

