What molecule am I?
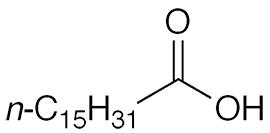

Palmitic acid, formally hexadecanoic acid, is a long-chain saturated fatty acid. It exists in nature primarily as a triglyceride and other esters. The most common natural fatty acid, it is abundant in oil extracted from the fruit of oil palms (Elaeis guineensis and E. oleifera), as well as in meats and dairy products.
The knowledge of palmitic acid dates to 1840, when French chemist Edmont Frémy made it by saponifying palm oil, a process that is still used to manufacture the compound. An early mention of palmitic acid in the chemical literature came in an 1879 report by Thomas Carnelley* and W. Carleton Williams at Owens College (Manchester, UK; eventually the University of Manchester), who measured the boiling point of its potassium salt, along with that of other compounds.
In the ensuing years of the 19th century, palmitic acid and its derivatives appeared in dozens of articles about its sources, properties, and uses. In an 1899 report on the chemistry of butterfat, C. A. Browne, Jr., at the Pennsylvania State College Agricultural Experiment Station lamented, “There is no problem in analytical chemistry more difficult than that of making a quantitative separation of a mixture of different fatty acids.”
Food sources that contain saturated fatty acid esters such as palmitic acid triglycerides and those of former Molecules of the Week lauric acid and myristic acid can lead to weight gain and increases in low-density lipoproteins and serum cholesterol. On the other hand, fatty acid esters are prolific producers of adenosine triphosphate (ATP), the main energy source in living cells. According to one account, one palmitate molecule generates 129 molecules of ATP.
For more information on palmitic acid, see the ScienceDirect topics page.
Palmitic acid hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Long-term (chronic) aquatic hazard, category 3 | H412—Harmful to aquatic life with long-lasting effects | |
*Compilation of multiple safety data sheets; some SDS state “not a hazardous substance or mixture”.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule in the News
2-Methoxyethanol1, known in commerce as Methyl Cellosolve, is an ether alcohol that is widely used as an industrial solvent. It was first described in 1891 by pioneering organic chemist William Henry Perkin, Jr., at Herriot-Watt College (Edinburgh), who synthesized it from hydroxyacetone2,3. Most 2-methoxyethanol is now produced from ethylene glycol and methanol.
Earlier this year, Brett A. McGuire and Zachary T. P. Fried at MIT (Cambridge, MA) and colleagues there and at other institutions in the United States, France, and Denmark, reported the discovery of 2-methoxyethanol in outer space. They first established the rotational spectrum of the molecule, then used the Atacama Large Millimeter/Submillimeter Array (ALMA; Chile) to detect it in the star-forming region NGC 6334I.
1. CAS Reg. No. 109-86-4.
2. CAS Reg. No. 116-09-6.
3. Perkin used the terms “methyl glycol” and “acetylcarbinol” for 2-methoxyethanol and hydroxyacetone, respectively.
Molecules in the News
MOTW highlights molecules that appear in major news outlets. See this week's edition.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule that you would like us to consider, please send us a message, preferably including links to references about your suggested molecule. And thank you for your interest in Molecule of the Week! —Ed.
Palmitic acid fast facts
| CAS Reg. No. | 57-10-3 |
| SciFindern name | Hexadecanoic acid |
| Empirical formula | C16H32O2 |
| Molar mass | 256.42 g/mol |
| Appearance | White crystals, flakes, or powder |
| Melting point | 64 ºC |
| Boiling point | 352 °C |
| Water solubility | 0.8 mg/L (30 ºC) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.


