The Invention of Warfarin
Dedicated at the University of Wisconsin-Madison, on Oct. 12, 2022
In Spanish: La invención de la warfarina
In Mandarin: 华法林的发明
In Arabic: اِختراع الوارفارين
“Warfarin,” the generic name for a prescription blood thinner first put on the market in the 1950s, might be familiar from television ads for more recent drugs. These ads claim that newer blood thinners, which are used to prevent strokes and other significant medical problems, are more convenient to take or have fewer side effects. Yet the ads mention its name because warfarin sets the standard as a globally respected, life-saving medicine that its latter-day competitors still aim to beat.
Warfarin’s contributions to science extend well beyond its use in human patients. First identified in a University of Wisconsin laboratory, warfarin is the most famous of a family of chemicals whose ability to prevent blood from clotting has served a variety of purposes — from keeping rodent populations in check, to treating a U.S. president after a heart attack.
Whether identified as dicumarol or warfarin sodium, by complex molecular formulas or the brand name Coumadin®, warfarin and its chemical cousins are life-changing chemicals that save lives when administered to prevent clotting. Warfarin is also a key part of an innovative program to use licensing revenue to fund future innovation.
Contents

View larger image

Agricultural beginnings
Warfarin had its origins on a Midwestern farm. During a blizzard in early 1933, a farmer named Ed Carlson drove more than 200 miles from Deer Park in northwest Wisconsin to the state capitol, Madison. He was looking for the state veterinarian to diagnose a condition that was killing his cows. Finding most offices closed on a Saturday, Carlson instead walked into the laboratory of Karl Paul Link, a professor at the University of Wisconsin campus in Madison (UW-Madison). Link and his student assistant, Eugen Wilhelm Schoeffel, examined what Carlson had brought with him — a milk jug full of blood, a dead cow, and a pile of moldy hay. The researchers recognized the signs of sweet clover disease: The cow’s blood would not clot, the animal hemorrhaged to death, and the moldy clover hay was to blame.
Sweet clover disease had become a persistent problem for cattle herds across the northern U.S. and southern Canada in the early 20th century. During the 1920s, two veterinarians, Frank W. Schofield in Ontario and Lee M. Roderick in North Dakota, traced the cause to wet, spoiled clover. Researchers continued to study the disease into the 1930s. Link first heard of the sweet clover problem from Ross A. Gortner at the University of Minnesota. Meanwhile at Wisconsin, geneticists Royal Alexander Brink and William K. Smith were studying coumarin— the chemical in clover that makes it smell sweet but taste bitter. As it turned out, naturally occurring coumarin would be important in solving the mystery of sweet clover disease.

Veterinary science informs biochemistry
Armed with the knowledge provided by his fellow scientists, but with much left to be discovered, Link and his assistants reoriented the work of their laboratory toward understanding the biochemistry at work in spoiled clover hay. They began by developing a bioassay — a test using small experimental animals — to study the chemical processes of coagulation and to determine how those processes interact with animal biology. By feeding various types of hay to rabbits and drawing their blood, the researchers hoped to learn what substance in spoiled clover acted as an anticoagulant.
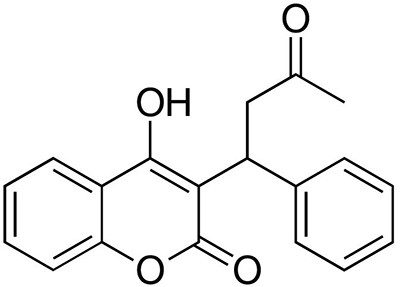
In 1939, after these experimental techniques were perfected, one of Link’s assistants, Harold A. Campbell, isolated a minuscule amount of a pure, crystalline substance that prevented blood from clotting. That substance was produced by a chemical reaction between coumarin and certain molds that grow when clover hay gets wet and spoils. Once Campbell isolated this new anticoagulant, his co-workers Mark A. Stahmann and Charles F. Huebner were able to determine its chemical structure: 3,3’-methylenebis(4-hydroxycoumarin).
In 1940, Stahmann and Huebner synthesized an identical substance in the laboratory, thereby verifying the molecule’s structure. Subsequently, Link and his coworkers synthesized more than 100 related compounds. Each of these analogs had a slight difference in chemical makeup, but all produced similar anticoagulant effects. The analog they labeled number 42, which was synthesized by Stahmann and Miyoshi Ikawa (another of Link’s assistants), would later come to be known as warfarin.
There is a lifesaving drug that owes its existence to moldy hay, sick cows, and rat poison. The drug is called warfarin sodium. It prevents blood clots, and it can be a lifesaver for patients who’ve had a heart attack or stroke. It’s one of the most widely prescribed drugs in the world.”
— “How Moldy Hay And Sick Cows Led To A Lifesaving Drug,” NPR’s “All Things Considered” program, Aug. 29, 2017
Biochemistry informs human medicine
At the time, Link and his coworkers had not determined which of the analogs was suitable for practical use — or for anything at all. But they had realized that whatever prevented cow blood from clotting might, in theory, also prevent dangerous blood clots in humans. The theory could only be verified through clinical trials, not in the laboratory. An idea that had traveled from the farm to the university would have to go to the hospital before reaching the shelves of the pharmacy.
Campbell, Stahmann, Huebner, and Link sought help in 1941 from the Wisconsin Alumni Research Foundation (WARF), a nonprofit that supports research and technology transfer at the University of Wisconsin. With WARF’s assistance, the team applied for a patent on their potential drug discovery. Over the following two decades, those four researchers, along with several coworkers, secured more than 20 patents on compounds, derivatives, and processes related to anticoagulation. WARF used the intellectual property protections provided by the patents to negotiate licenses with companies equipped to develop and commercialize the patents.
The first WARF patent covered the compound synthesized in 1940. Scientists named it “dicumarol,” and it entered human trials in 1941 at the Wisconsin General Hospital and the Mayo Clinic. Over the ensuing decade, the analogs that followed dicumarol showed varying levels of potency and different effects in different species of animals.
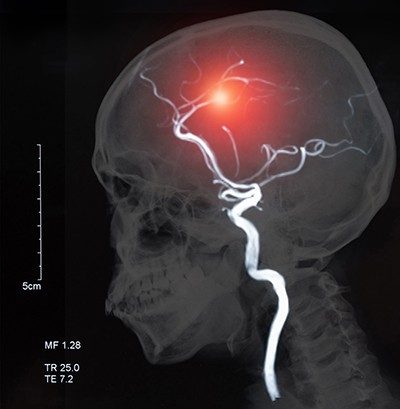
Into the future
Some of the analog compounds, especially number 42, were lethal to small animals, to the point that some of the Wisconsin scientists were concerned it might give dicumarol a reputation of being dangerous. WARF nonetheless forged ahead with patenting the analogs and, with timely assistance from Stahmann, managed to file an application for analog 42 less than a month before patent eligibility would have expired. The patent, held jointly by Link, Stahmann, and Ikawa, proved fortuitous. Subsequent tests showed that, while analog 42 would indeed kill mice and rats, it was not toxic enough to pose any significant danger to larger animals, such as dogs, cats, or even curious toddlers.
With the possibilities for pest control now clear, Link decided it would need a catchier name. He combined the acronym WARF with the last syllables of coumarin to come up with “warfarin.” With its new moniker secured, number 42 entered the market in 1948 — but only as a rat poison.
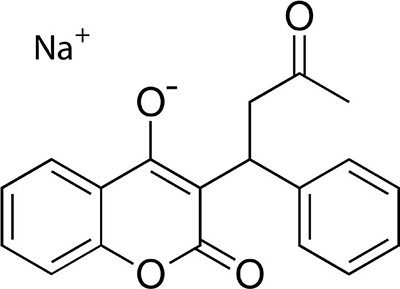
In the meantime, research continued on all of the anticoagulant compounds to identify the safest and most effective as a blood thinner in human patients. Dicumarol showed some success as a treatment option but was unstable and cumbersome when administered in hospitals. Some further tinkering with the warfarin formula then produced an ironic result: The most effective analog at killing rats also proved to be the safest and most effective for preventing blood clots in people. A water-soluble version, known as warfarin sodium, could be administered orally or intravenously and could be managed with routine, follow-up blood tests, rather than the close monitoring required for dicumarol.
Warfarin sodium was approved for human use in 1954 and went on the market under the brand name Coumadin. It soon became both the most widely used rat poison and the most widely prescribed blood thinner in the world. The widespread commercial success allowed WARF to use the proceeds from its warfarin patents to invest $16 million (or $144 million in 2022 dollars) into the research enterprise at UW-Madison.
The association of the name “warfarin” with rat poison did lead to some hesitancy among doctors to recommend it and among patients to take it, but those fears were soon overcome. In 1955, word got out that U.S. President Dwight D. Eisenhower had been given warfarin following a heart attack. Continued clinical testing had already persuaded doctors it was the best available option, and the effective treatment of a world leader helped put patients’ minds at ease (as did the more frequent use of Coumadin as a brand name in place of warfarin).
In any event, as the years went by, warfarin sodium became less effective as a poison as rodent populations developed resistance. Scientists responded by developing a second generation of anticoagulant compounds (including brodifacoum, difenacoum, bromadiolone, and difethiolone), which came to be called “superwarfarins.” These more advanced chemicals are not given to humans.
Research continued on the medical front as well. In the 2010s, new pharmaceuticals categorized as “novel oral anticoagulants” or “direct oral coagulants” entered the market. Sold under brand names such as Pradaxa®, Xarelto®, Eliquis®, and Savaysa®, these drugs require less invasive medical testing and cause fewer side effects compared to warfarin. On the other hand, they also tend to be more expensive and lack the decades of clinical data and safety testing of a drug used since the 1950s.
Prescription rates for warfarin are declining, but it remains one of the most widely used treatments for blood clots to this day. Experts estimate that around 100 million prescriptions of warfarin are still issued globally each year.
Warfarin is still used to treat millions of people. In fact, it remains on the World Health Organization’s list of essential medicines.”
— “Blood, rats and anticoagulants: The story of warfarin,” Nature video, April 1973

View larger image
Landmark dedication and acknowledgments
Landmark dedication
The American Chemical Society (ACS) honored the development of the blood thinner warfarin with the National Historic Chemical Landmark (NHCL) designation in a ceremony at the University of Wisconsin-Madison, on Oct. 12, 2022. The commemorative plaque reads:
In the early 20th century, a devastating bleeding disorder swept through North American cattle herds. Focusing on the suspected spoiled hay in the animals’ food supply, University of Wisconsin biochemists in 1939 isolated a chemical compound that prevents blood from clotting. With support from the university, state and Wisconsin Alumni Research Foundation (WARF), further research on this compound and its analogs led to the development of a revolutionary new rat poison (warfarin) and blood thinner (warfarin sodium) still widely used today. These anticoagulant compounds have reduced rodent-borne diseases and helped millions of patients lessen the risk of stroke or heart attack. Proceeds from the associated patents are important contributors to the over $4 billion in research funded by WARF at the University of Wisconsin-Madison.
Acknowledgments
Written by Kevin Walters.
The author wishes to thank contributors to and reviewers of this booklet, all of whom helped improve its content, especially members of the ACS NHCL Subcommittee.
The nomination for this Landmark designation was prepared by the ACS Wisconsin Section and the Wisconsin Alumni Research Foundation.

Booklet PDF

