What molecule am I?

Many of you are familiar with “sandwich” compounds in which a metal atom is complexed between two five- to eight-membered aromatic rings. Early examples are ferrocene [Fe(cp)2; cp is cyclopentadienyl] and bis(benzene)chromium.
As many other sandwich compounds were being synthesized, chemists began to make “half-sandwich” or “piano-stool” compounds. The latter term arose because the molecular structure resembles a stool in which an arene is the seat and the opposite ligands are the legs. One useful compound in this category is methylcyclopentadienylmanganese tricarbonyl, which has been used as a replacement for tetraethyllead to increase the octane rating of gasoline.
In the past 20 years or so, researchers have been synthesizing piano-stool complexes in which the “legs” are amino acids. In addition to having properties similar to those of other half-sandwiches, the amino acid ligands in these species have a propensity to bridge to other complexes and oligomerize into polynuclear rings. In particular, a piano-stool complex that consists of a metal with cyclopentadienide, amino acid, and chloride ligands, when treated with a poorly coordinating anion such as tetrafluoroborate (BF4–) to displace the chloride, forms a trinuclear compound.
At least, that’s what chemists thought until Joseph S. Merola and colleague David Morris at Virginia Tech (Blacksburg) began to experiment with different metals, amino acids, and anions.
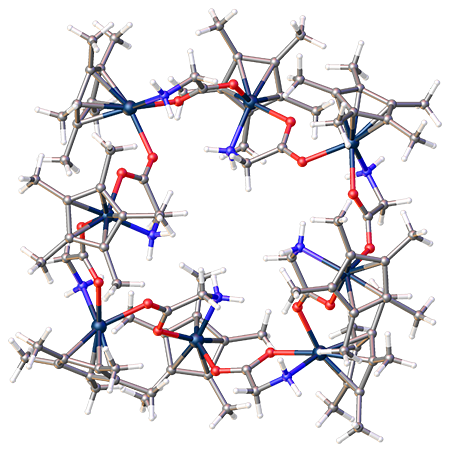
When the researchers chose iridium as the metal, pentamethylcyclopentadienide (Cp*) as the arene, and glycine as the amino acid [formula Cp*Ir(glycinato)Cl] and displaced chloride with BF4–, the product was the normal three-unit complex. But when they switched BF4– to hexafluorophosphate (PF6–) as the displacing anion, an unprecedented octametallic structure formed (see figures). The authors explain that the way that PF6– is encapsulated within the nascent complex is responsible for their unexpected finding.
Image credits: 3-D structure, Joseph S. Merola; octopus drawing, Christine DuChane.
Octamer of iridium complex cations fast facts
| CAS Reg. No. | 2396760-17-9 |
| SciFinder nomenclature | Not yet assigned |
| Empirical formula | C96H152Ir8N8O16∙8F6P |
| Molar mass | 4371.72 g/mol |
| Appearance | Clear pale yellow crystals |
| Melting point | Decomposes |
| Water solubility | Solublea |
a. Dissolves with decomposition into components.


Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

