The Periodic Table
Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts
- The periodic table is a chart containing information about the atoms that make up all matter.
- An element is a substance made up of only one type of atom.
- The atomic number of an atom is equal to the number of protons in its nucleus.
- The number of electrons surrounding the nucleus of an atom is equal to the number of protons in its nucleus.
- Different atoms of the same element can have a different number of neutrons.
- Atoms of the same element with different numbers of neutrons are called “isotopes” of that element.
- The atomic mass of an element is the average mass of the different isotopes of the element.
- The atoms in the periodic table are arranged to show characteristics and relationships between atoms and groups of atoms.
Summary
Students will begin to look closely at the periodic table. They will be introduced to the basic information given for the elements in most periodic tables: the name, symbol, atomic number, and atomic mass for each element. Students will focus on the first 20 elements. They will try to correctly match cards with information about an element to each of the first 20 elements. Students will then watch several videos of some interesting chemical reactions involving some of these elements.
Objective
Students will identify different atoms by the number of protons in the nucleus and realize that the number of electrons equals the number of protons in a neutral atom. They will also be able to explain the meaning of atomic number and atomic mass.
About this Lesson
Lessons 2 and 3 both use the 20 sheets of atom description cards – one sheet for each element.
Teacher preparation
Print out the 20 pages of element cards. The first page is shown. Laminate each page and cut out the cards. For Lesson 2, you will need the 5 cards for each element from the left side of each sheet. You will also need the card in the upper right corner. This is the atom name card. Tape each of the 20 atom name cards to a spot in the room where students can place the cards that match that atom nearby. For Lesson 3, you will need the atom name card, taped in the same location in the room, and the four cards beneath it. Divide the class into 10 groups of 2 or 3 students each.
Print out the Element Cards and play the Periodic Table Game, Game #1.
- Lesson 4.2 and 4.3 Element Cards PDF
Students can also try playing the Periodic Table Game, Game #1. This is an online version of the periodic table card game from this lesson that you can assign as class work or homework after students have played the game in the classroom.
Download All Lesson 4.2 Resources
Get the entire lesson plan and Student Activity Sheet for "Lesson 4.2 - The Periodic Table."
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Instructions
1 Engage
Step 1
Introduce students to the periodic table.
Project the image Periodic Table.
Tell students that this is the periodic table. Explain that each box contains information about a different atom. The periodic table shows all the atoms that everything in the known universe is made from. It’s kind of like the alphabet in which only 26 letters, in different combinations, make up many thousands of words. The 100 or so atoms of the periodic table, in different combinations, make up millions of different substances.
Note: It is often confusing for students to see the terms “atom” and “element” used interchangeably as if they are the same thing. Explain to students that an atom is the smallest particle or “building block” of a substance. An element is a substance made up of all the same type of atom. For instance, a piece of pure carbon is made up of only carbon atoms. This piece of pure carbon is a sample of the element carbon. The people who developed the periodic table could have called it the Periodic Table of the Atoms but they did not have a firm understanding of atoms at that time. Since they were working with actual samples of elements such as copper, mercury, sulfur, etc., they called it the periodic table of the elements.
Optional
Play one or both of the following songs.
Step 2
Explain the meaning of the numbers and letters in the boxes in the periodic table.
Tell students that the class will focus on the first 20 elements over 2 days. On the first day, they will look at the number of protons, electrons, and neutrons in the atoms of each element. On the second day, they will look at the arrangement of electrons in the atoms.
Give each student a copy of the periodic table of the elements, the periodic table of elements 1–20, and the activity sheet.
Students will use the periodic table of elements 1–20, along with the activity sheet, in the lesson they will do today.
- Lesson 4.2 Student Activity Sheet PDF | DOCX | Google Doc
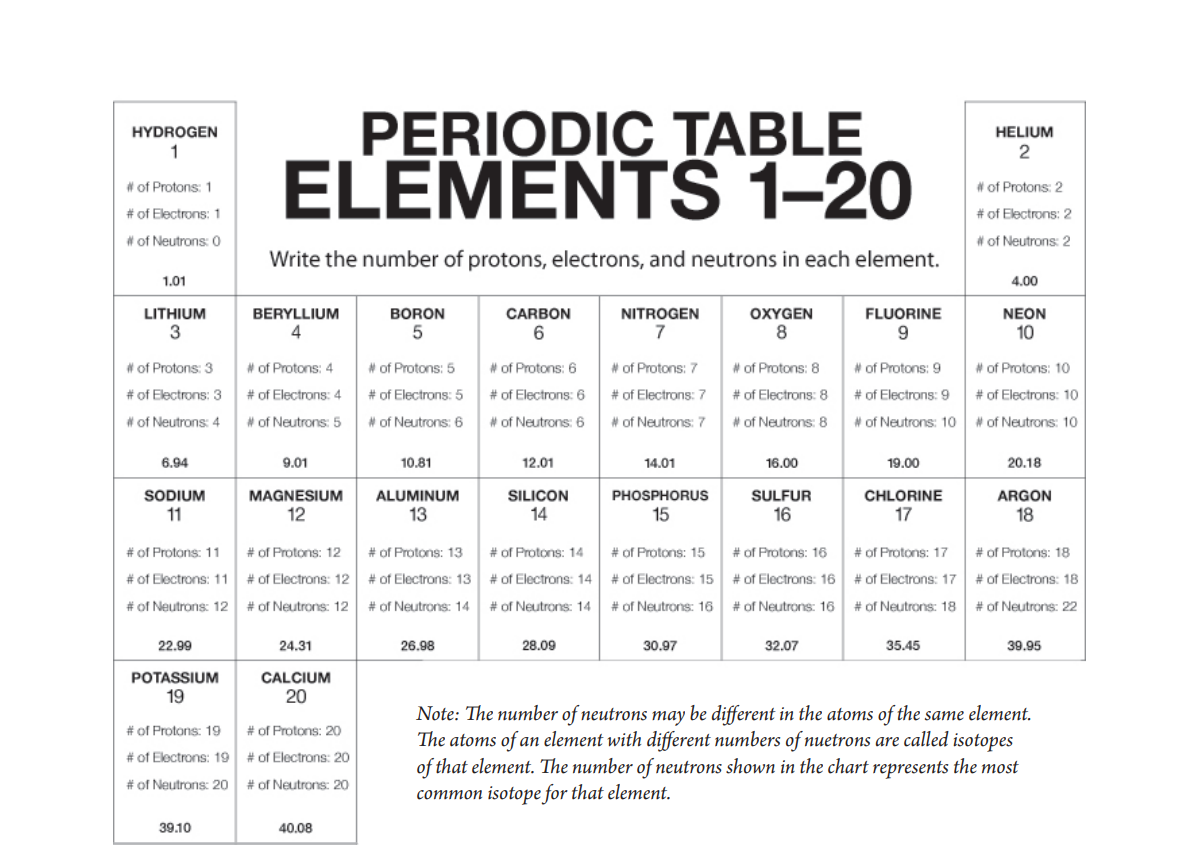
Project the image Periodic Table of the First 20 Elements.
Project the image Element explanation.
Explain what the numbers and letters in each box on the periodic table represent.
Explain atomic mass.
The atomic mass of an element is based on the mass of the protons, neutrons, and electrons of the atoms of that element. The mass of the proton and neutron are about the same, but the mass of the electron is much smaller (about 1/2000 the mass of the proton or neutron). The vast majority of the atomic mass is contributed by the protons and neutrons.
For any element in the periodic table, the number of electrons in an atom of that element always equals the number of protons in the nucleus. But this is not true for neutrons.
Atoms of the same element can have different numbers of neutrons than protons. Atoms of the same element with different numbers of neutrons are called isotopes of that element. The atomic mass in the periodic table is an average of the atomic mass of the isotopes of an element. For the atoms of the first 20 elements, the number of neutrons is either equal to or slightly greater than the number of protons.
For example, the vast majority of carbon atoms have 6 protons and 6 neutrons, but a small percentage have 6 protons and 7 neutrons, and an even smaller percentage have 6 protons and 8 neutrons. Since the majority of carbon atoms have a mass very close to 12, and only a small percentage are greater than 12, the average atomic mass is slightly greater than 12.
Step 3
Describe the activity students will do to learn about the first 20 elements of the periodic table.
Show students that you have 100 cards (5 for each of the first 20 elements). Explain that each card contains information about one of the first 20 atoms of the periodic table. The students’ job is to read the card carefully, figure out which atom the card is describing, and put the card at the spot in the room for that atom.
Review the information about protons, electrons, and neutrons students need to know in order to match the cards with the correct element:
Proton
- Positively charged particle in the nucleus of the atom.
- The number of protons in an atom’s nucleus is the atomic number.
Electron
- Negatively charged particle surrounding the nucleus of the atom.
- The number of electrons surrounding the nucleus of an atom is equal to the number of protons in the atom’s nucleus.
Neutron
- Particle in the nucleus that has almost the same mass as a proton but has no charge.
- For the atoms of the first 20 elements, the number of neutrons is either equal to or slightly greater than the number of protons.
To match the number of neutrons listed on your card to the correct element, look for an element on the periodic table so that if you add the number of neutrons on your card to the protons of the element, you will get close to the atomic mass for that element.
For example, you may have a card that says that the atom you are looking for has 5 neutrons. You would look at the periodic table to find an atom that you could add 5 to its number of protons that would give you a sum close to the atomic mass given for that element. The answer is beryllium (Be), which has 4 protons and an atomic mass of 9.01.
Note: There are a few neutron cards that have two possible correct elements instead of just one:
- 6 Neutrons—Boron or Carbon
- 10 Neutrons—Fluorine or Neon
- 12 Neutrons—Sodium or Magnesium
- 16 Neutrons—Phosphorous or Sulfur
- 20 Neutrons—Potassium or Calcium
2 Evaluate
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding.
- Lesson 4.2 Student Activity Sheet PDF | DOCX | Google Doc
- Lesson 4.2 Activity Sheet Answers PDF | DOCX | Google Doc
3 Explore
Step 4
Have groups work together to place each card with its correct atom.

Distribute the cards to groups. If you have 10 groups, each group will get 10 cards. Be available to help students who have trouble with the neutrons and atomic mass.
Step 5
Discuss the placement of the cards for two or three atoms.
Select two or three atoms and review whether the cards were placed correctly. This review will help reinforce the concepts about the structure of atoms and help students determine the number of protons, electrons, and neutrons in each type of atom.
Have students begin filling out the activity sheet with the following information:
- Number of protons
- Number of electrons
- Number of neutrons (usually)
4 Extend
Step 6
Introduce students to their element project and an online resource that they can use.
Have students work in pairs and assign each pair one of the 20 elements.
Students should find and present some basic information about their element to the class. The presentation can be in the form of a poster, pamphlet, PowerPoint presentation or other form. The presentations should be short and can include: atom name, atomic number, derivation of name, natural sources of the element, major uses, and any other information you find important.
Some online periodic tables can be overwhelming for middle school students. This colorful Interactive Periodic Table of the Elements contains enough information and resources on the first 20 elements to be useful for students but not intimidating with an overload of technical information.
If there is time available, have students work on this atom project during the week.
Periodic Table Elements 1-20
Videos, illustrations, and information about the first 20 elements of the periodic table.
*Part of Lesson 4.2
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Simulations
Periodic Table Elements 1-20
Videos, illustrations, and information about the first 20 elements of the periodic table.
*Part of Lesson 4.2
Downloads
For Students
- Lesson 4.2 Student Activity Sheet PDF | DOCX | Google Doc
- Lesson 4.2 Element Cards PDF
For Teachers
- Lesson 4.2 Lesson Plan PDF | DOCX | Google Doc
- Lesson 4.2 Activity Sheet Answers PDF | DOCX | Google Doc
- Lesson 4.2 and 4.3 Element Cards PDF
Resources for the entire Chapter 4
- Chapter 4 Student Reading PDF | DOCX | Google Doc
- Chapter 4 Test Bank PDF | DOCX | Google Doc
Interactive Lesson Modules
- Lesson 4.2 Online Assignments Google Form
Have Questions? Visit Help Center




