A Catalyst and the Rate of Reaction
Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts
- A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster.
- A catalyst does not actually become part of the products of the reaction.
Summary
Students watch a video and do a quick activity to see that a catalyst can increase the rate of the breakdown (decomposition) of hydrogen peroxide. Students will then use salt as a catalyst in a reaction between aluminum foil and a solution of copper II sulfate. Students will be introduced to the concept that a catalyst increases the rate of a chemical reaction but is not incorporated into the products of the reaction.
Objective
Students will be able to define a catalyst as a substance that increases the rate of a chemical reaction but is not incorporated into the products of the reaction.

Safety
Be sure you and the students wear properly fitting goggles. When using hydrogen peroxide, follow all warnings on the label. After students have conducted the activity with the copper II sulfate solution and aluminum foil, allow the contents of the cup to evaporate. Put the small amount of solid in a paper towel and dispose in the trash or use a disposal method required by local regulations.
Materials for Each Group
- Graduated cylinder (50 mL or 100 mL)
- Hydrogen peroxide (3%)
- Yeast
- 2 Popsicle sticks
- Detergent solution
- Dropper
- Small cup
- Clear plastic cup
- Copper II sulfate solution (in cup)
- Salt
- Aluminum foil (5 cm × 5 cm)
- Thermometer
Download All Lesson 6.5 Resources
Get the entire lesson plan and Student Activity Sheet for "Lesson 6.5 - A Catalyst and the Rate of Reaction."
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Instructions
1 Engage
Step 1
Show students two demonstrations and have them look for evidence that a gas is produced in the chemical reactions.
Tell students that you will show them videos of two demonstrations where water vapor and oxygen gas are produced in the exact same chemical reaction. Because gases are invisible, ask students to watch closely for evidence that a gas is produced.
Project the video Elephant’s Toothpaste.
The foaming shows that gases (oxygen and water vapor) are being produced very quickly. The amount of foam produced in a period of time is a way of measuring the rate of the reaction.
Project the video Genie in a Bottle.
The steam coming out of the bottle is water vapor that is condensing as it leaves the bottle. Oxygen is also leaving the bottle, but it is invisible.
Ask students:
- How could you tell that a gas is produced in the chemical reaction?
The foaming in the elephant toothpaste demonstration means that a gas is produced. Production of a gas is a clue that a chemical reaction has occurred. The water vapor in the genie-in-a-bottle demonstration also shows the production of a gas.
Tell students that this lesson is about speeding up chemical reactions. Some reactions occur very slowly, but chemicals called catalysts can be added in order to make them hap- pen faster. Both of these demonstrations relied on a catalyst.
2 Evaluate
Give each student an activity sheet.
- Lesson 6.5 Student Activity Sheet PDF | DOCX | Google Doc
- Lesson 6.5 Activity Sheet Answers PDF | DOCX | Google Doc
Download the student activity sheet, and distribute one per student.
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms and Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
3 Explain
Step 2
Describe how the decomposition of hydrogen peroxide produced oxygen gas in both of the videos.
Tell students that both demonstrations use a 30% hydrogen peroxide solution. Typically, the hydrogen peroxide you can buy at the store is only 3% hydrogen peroxide.
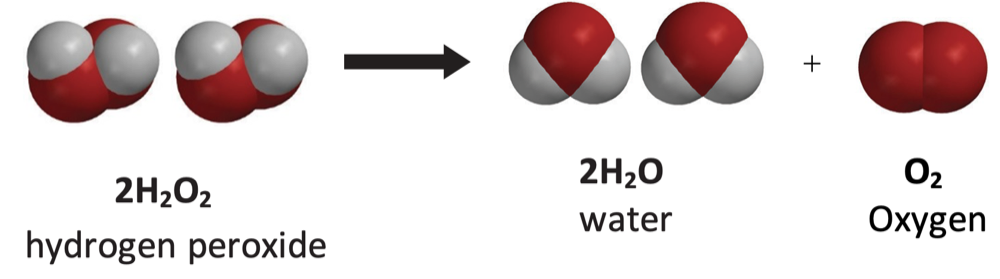
Explain to students that the chemical formula for hydrogen peroxide is H2O2. Point out that hydrogen peroxide is not very stable and breaks down into water and oxygen. This kind of change is a chemical reaction called decomposition. The decomposition of hydrogen peroxide is slow and is not usually noticeable.
Project the image Decomposition of Hydrogen Peroxide.
Explain that hydrogen peroxide decomposes to form water and oxygen according to this chemical equation:
4 Explore
Step 3
Have students use yeast to catalyze the decomposition of hydrogen peroxide.
Question to Investigate
Can another substance catalyze the decomposition of hydrogen peroxide?
Materials for Each Group
- Graduated cylinder
- Hydrogen peroxide (3%)
- Yeast
- Popsicle stick
- Detergent solution
- Dropper
Teacher Preparation
Make a detergent solution by adding 1 teaspoon of liquid dish detergent to 2 tablespoons of water. Divide this detergent solution equally into one small cup for each group.
Procedure
- Add 10 mL of hydrogen peroxide to a graduated cylinder. Add 1 drop of detergent solution. Swirl gently and watch the solution for any bubbling.
Explain to students that the detergent is added only to make bubbles if any gas is produced. Since the breakdown of hydrogen peroxide produces oxygen gas, bubbling shows that the hydrogen peroxide is breaking down or decomposing. The lack of bubbling shows that not much oxygen gas is being produced.

- Use the end of a popsicle stick to add a small amount of yeast to the hydrogen peroxide in the graduated cylinder and swirl.
- Place the graduated cylinder on the table and watch for any bubbling.
- Hold the graduated cylinder to see if there seems to be any change in temperature.
Expected Results
Before the yeast is added, there is no observable bubbling. After the yeast is added, bubbling will cause foam to move up the graduated cylinder. Also, the graduated cylinder should feel a little warmer because the decomposition of hydrogen peroxide releases energy. Energy changes in chemical reactions will be investigated in more detail in Chapter 6, Lesson 7. Discuss student observations.
Ask students:
- What clues did you have that a chemical reaction occurred in this activity?Bubbling. Tell students that a change in temperature is also a sign that a chemical reaction may be occurring. Endothermic and exothermic chemical reactions will be addressed in Chapter 6, Lesson 7.
- What is the catalyst in this activity?
A substance in yeast. - What evidence do you have that hydrogen peroxide decomposed faster when you added yeast?
Bubbles of oxygen gas were produced after the yeast was added. - When you write the chemical equation for this reaction, should yeast be included on the product side of the chemical equation?
Explain to students that the catalyst in the yeast does not end up in the products but is a substance that helps the decomposition happen faster. Sometimes a catalyst is written above or below the arrow in a chemical equation, but it is never included with the reactants or products.
In general, catalysts work by providing a place where reactants can come together to react. Explain to students that cells in yeast and other organisms contain a catalyst called catalase. Through normal cell processes, living things produce hydrogen peroxide in their cells. But hydrogen peroxide is a poison, so the cells need a way to break it down very quickly. The catalase in the cell breaks down hydrogen peroxide at a very fast rate. A single molecule of catalase can catalyze the breakdown of millions of hydrogen peroxide molecules every second.
Students may continue to explore the effect of catalase on hydrogen peroxide by adding a piece of raw fresh potato to a small amount of hydrogen peroxide.
5 Extend
Step 4
Have students identify the changes that occur when copper II sulfate reacts with a piece of aluminum foil.
Note: This is a reaction between copper II sulfate and aluminum. The copper is called “copper II” because copper can make different types of ions. It can lose one electron and be just Cu+ or it can lose two electrons and be Cu2+. This type of copper ion is called copper II. Also, the “sulfate” in copper II sulfate is also an ion. This ion is made up of more than one atom. It is one of the polyatomic ions discussed in Chapter 4, Lesson 3. The sulfate is made up of a sulfur atom bonded to four oxygen atoms and is treated as one ion (SO42-).
There are several interesting aspects of the reaction between copper II sulfate and aluminum, but it is different from the other reactions students have seen so far. In this reaction, the movement of electrons, rather than entire atoms, ions, or molecules, causes the reaction to occur. This type of reaction is called an oxidation/reduction reaction. This particular reaction is fun to do because it is exothermic, generates a gas, and copper metal appears as aluminum metal disappears.
Salt can be considered a catalyst in the reaction but has a different role than most catalysts. Copper II sulfate and aluminum react very slowly because aluminum is coated with a very thin layer of tarnish (aluminum oxide). This reaction can be sped up if the layer of aluminum oxide is removed or compromised. Adding salt does this and allows electrons from the aluminum to react with the copper ions in the solution, causing them to become copper metal.
Question to Investigate
What is the catalyst in the following activity?
Materials for Each Group
- Copper II sulfate solution (in cup)
- Clear plastic cup (empty)
- Salt
- Piece of aluminum foil
- Thermometer
- Popsicle stick
Teacher preparation
Make a copper II sulfate solution by adding 20g of copper II sulfate to 200 mL of water. Pour about 25 mL of copper II sulfate solution into a cup for each group. Cut aluminum foil into pieces big enough to cover the bottom of a cup (about 5 cm long × 5 cm wide).
Procedure
- Place the piece of aluminum foil in an empty cup. Use your fingers or a Popsicle stick to push the foil firmly down so that it lays flat and covers the bottom of the cup.
- Add all the copper II sulfate solution to the cup with the aluminum foil.
- Gently swirl the solution for a few seconds
- and let it stand still. Watch the aluminum for any bubbling or color change.
- Use your Popsicle stick to place a small amount of salt in the copper II sulfate solution. Gently swirl the solution for a few seconds and let it stand still. Watch for any bubbling or color change.
- Carefully place a thermometer in the cup and see if the temperature changes.
Expected Results
Before the salt is added, there is no bubbling or color change. After the salt is added, the color turns greenish, and bubbles begin to form on the aluminum. Soon, brownish material (copper) begins to form on the aluminum. The bubbling becomes more vigorous, and the solution loses its blue color as the aluminum disappears and more copper is produced. The solution also gets warmer.
Step 5
Discuss student observations.
Ask students:
- How do you know that a chemical reaction occurs when aluminum foil and sodium chloride are placed in copper II sulfate solution?
There was bubbling, a color change, an increase in temperature, and a different solid was formed. - What is the catalyst in this activity?
Salt. - How is adding salt to the aluminum similar to adding yeast to the hydrogen peroxide?
Both can be seen as catalysts. Adding yeast helps the hydrogen peroxide decompose faster and adding salt helps the aluminum react with the copper II sulfate.
Tell students that the blue solution contains copper ions (Cu2+). Adding salt to the solution helps remove a layer of tarnish from the piece of aluminum in the solution. This exposes some aluminum and allows electrons from the aluminum to react with the copper ions. These negative electrons are attracted to the positive copper ions. When the electrons join with the copper ions, the ions become neutral copper atoms and look like copper metal in the solution. As the aluminum loses its electrons, it becomes aluminum ions which go into the solution.
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Downloads
For Students
- Lesson 6.5 Student Activity Sheet PDF | DOCX | Google Doc
For Teachers
- Lesson 6.5 Lesson Plan PDF | DOCX | Google Doc
- Lesson 6.5 Activity Sheet Answers PDF | DOCX | Google Doc
Resources for the entire Chapter 6
- Chapter 6 Student Reading PDF | DOCX | Google Doc
- Chapter 6 Test Bank PDF | DOCX | Google Doc
Interactive Lesson Modules
- Lesson 6.5 Online Assignments Google Form
Have Questions? Visit Help Center