Using Chemical Change to Identify an Unknown
Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts
- Substances react chemically in characteristic ways.
- A set of reactions can be used to identify an unknown substance.
Summary
Students will use test liquids on different known powders and observe their reactions. Then students will use these characteristic chemical changes to help them identify an unknown powder.
Objective
Students will be able to identify and control variables to develop a test to identify an unknown powder. Students will be able to explain that a substance reacts chemically in characteristic ways and that these characteristics can be used to identify an unknown substance.

Safety
Be sure you and the students wear properly fitting goggles. When using tincture of iodine, follow all warnings on the label. Have students wash hands after the activity.
Materials for the Demonstrations
- Baking soda
- Cornstarch
- Cream of tartar
- Tincture of iodine
- Vinegar
- Water
- Universal indicator
- Graduated cylinder or beaker
- 2 droppers
- ¼ teaspoon
- 5 clear plastic cups
- 3 Popsicle sticks
Materials for Each Group
- Baking soda
- Baking powder
- Cream of tartar
- Cornstarch
- Water
- Vinegar
- Tincture of iodine
- Universal indicator
- 10 small plastic cups
- 4 droppers
- 8 Popsicle sticks
- Testing chart (laminated or covered with wax paper)
Download All Lesson 6.6 Resources
Get the entire lesson plan and Student Activity Sheet for "Lesson 6.6 - Using Chemicl Change to Identify an Unknown."
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Instructions
1 Engage
Step 1
Add iodine solution to baking soda and cornstarch to introduce the idea that different substances react chemically in characteristic ways.
Materials for the Demonstration
- Tincture of iodine
- Baking soda
- Cornstarch
- Water
- Graduated cylinder or beaker
- 2 Popsicle sticks
- Dropper
- ¼ teaspoon
- 3 clear plastic cups
Teacher Preparation
- Make a dilute tincture of iodine solution by adding about 10 drops of tincture of iodine to 100 mL of water. Pour 50 mL of this solution into a clear plastic cup for this demonstration.
- Set the other 50 mL aside for the student activity. Instructions for preparing the rest of the materials for the student activity are in the Explore section of this lesson.
- Place ¼ teaspoon corn starch in a clear plastic cup and ¼ teaspoon baking soda in another cup. Do not tell students which powder is in each cup.

Procedure
- Tell students that you have a different powder in each cup. Both are white and look alike, but they are chemically different.
- Pour about 25 mL of the iodine solution in each cup and swirl.
Expected Results
The iodine solution stays light brown when added to the baking soda. The iodine solution and corn starch turns a very dark purple.
Ask students:
- Both powders looked similar at first. How do you know that they are different?
The iodine changed color in one powder, but not in the other. - Which do you think is probably a chemical change?
The iodine and the cornstarch are probably the chemical change because the dramatic color change seems like something new may have been produced. The iodine does not change color when it combines with the baking soda. - What other tests could you conduct with baking soda and cornstarch to compare their characteristic properties?
Let students know that there can be no tasting or smelling of the powders. Students might suggest adding water to see if they dissolve differently or maybe adding another substance to see if a different chemical reaction takes place.
2 Evaluate
Give Each Group a Testing Chart.
Make one copy of the testing chart, found at the end of the downloaded activity sheet, for each group. Either laminate this testing chart or have students lay a piece of wax paper over it.
Give each student an activity sheet.
- Lesson 6.6 Student Activity Sheet PDF | DOCX | Google Doc
(Includes Testing Chart) - Lesson 6.6 Activity Sheet Answers PDF | DOCX | Google Doc
Download the student activity sheet, and distribute one per student.
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms and Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
3 Explore
Step 2
Introduce the activity and ask students how they might test and compare the four different powders with four different test solutions.
Tell students that in this activity they will test four different similar-looking powders with four different test solutions. The four powders are baking soda, baking powder, cream of tartar, and cornstarch. The four test solutions are water, vinegar, iodine solution, and universal indicator.
Explain that each powder will react in a certain way with each solution used to test it. Each powder and solution pair is one set of reactants. Let students know that in some cases, no chemical reaction will occur.
Students will need to observe and record the reactions the liquids have with each powder.
Have students look at the testing chart. Point out that the names of the four test solutions are on the left and the names of the different powders are on the top.
There is also one column for an unknown powder. Explain that after testing all four known powders and recording their observations, you will give students an unknown powder to identify.
Ask students questions like the following to help them plan how they will organize and conduct their tests:
- Do we need more than one pile of each powder placed on the chart?
Yes. Each powder will be tested with each of the four solutions so there needs to be four piles of each powder in the squares under its name. - Should all the squares on the entire chart have samples of powder on them before you start testing?
It is best if students place the four samples of one particular powder in its column on the testing sheet. Then students should test that powder with each of the four solutions. Students should test a single powder with each of the test liquids before moving on to the next powder. - Do the piles have to be about the same size?
The size of the piles is not particularly important as long as enough powder is used to see a reaction, if there is one. When testing the unknown, try to make the piles of unknown about the same size as the piles of the other powders. - Should the number of drops placed on each pile be the same?
The precise number of drops is not particularly important, although enough liquid should be added to see if there is a reaction. When testing the unknown, be sure that the number of drops used on the unknown is the same as the number used on the other powders. - How will you remember your observations for each reaction?
Students should record their observations immediately after a single test solution is added to a powder. These will be recorded in a chart in the corresponding box on the activity sheet.
Step 3
As an example, guide the class as they test baking soda with water, vinegar, iodine solution, and universal indicator.
Question to Investigate
Can you use the characteristic ways substances react to tell similar-looking substances apart?
Materials for Each Group
- Baking soda in cup
- Baking powder in cup
- Cream of tartar in cup
- Cornstarch in cup
- Water in cup
- Vinegar in cup
- Tincture of iodine solution in cup
- Universal indicator solution in cup
- 4 Popsicle sticks
- Testing chart, either laminated or with a piece of wax paper over it
- 4 droppers
Teacher Preparation
Each group will need five labeled cups each containing one of the powders and four labeled cups each containing one of the four test solutions to complete all three of the activities in this lesson.
Prepare the powders
- Label five small cups Baking Soda, Baking Powder, Cream of Tartar, Cornstarch and Unknown.
- Place about ½ teaspoon of each powder into its labeled cup. These powders will be tested in this activity.
- Place about ½ teaspoon of baking powder in the cup labeled unknown. Place the unknown cups aside. Students will test this unknown powder after they have tested each of the “known” powders and recorded their observations.
Prepare the Test Solutions
- Label four cups Water, Vinegar, Iodine, and Indicator.
- Use the iodine solution left over from the demonstration or make a new solution by adding 5 drops of tincture of iodine to 50 mL of water. Place about 5 mL iodine solution in a small, labeled cup for each group.
- Make a universal indicator solution by adding 5 mL of universal indicator to 100 mL of water. Place about 5 mL (or 1 teaspoon) indicator solution in a small, labeled cup for each group. Some indicator solution will be left over for the demonstration at the end of the lesson.
- Place about 5 mL of water and vinegar into their small, labeled cups.
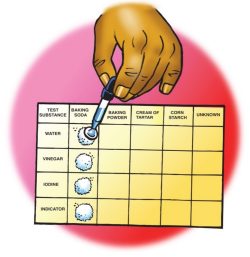
Procedure
- Use the end of a popsicle stick to place four equal piles of baking soda on the testing chart in the baking soda column. Let students know that they should not use all the powder at this time. The remaining powder will be used in the Extend portion of this lesson.
- Add 5 drops of water to the first pile of baking soda. Record your observations in the chart on the activity sheet.
- Continue testing each pile of baking soda with a different test solution and recording your observations.
Expected Results
There will be no change with water, bubbling with vinegar, and little to no change with the iodine or indicator solutions.
Ask students:
- What did you observe when each test solution was added to a sample of baking soda?
Baking soda bubbles with vinegar. There is no change with water or iodine solution. Students may see a slight color change with the indicator solution. Explain to students that their results show them the characteristic set of reactions that baking soda has with these four test solutions. - Would you expect each test solution to react with baking powder the same way as it did with baking soda?
No. Each powder should have a different set of reactions.
Have students conduct the tests on the remaining powders and record their observations.
Procedure
- Test each of the powders with the test solutions the way you tested baking soda and record your observations.
Expected Results
| Test Solution | Baking Soda | Baking Powder | Cream of Tartar | Cornstarch |
| Water | No change | Bubbling | No change | No change |
| Vinegar | Lots of bubbling that ends quickly | Bubbling | No change | No change |
| Iodine | No change | Bubbling, purple | Orange | Purple |
| Indicator | Greenish-blue | Bubbling, orange changes to yellow with some green | Dark orange or pink | Brighter green, may have some orange |
Step 4
Give students the unknown powder and have them use their test solutions and observation chart to identify it.
Give each group the unknown powder. Explain to students that the unknown is one of the four powders they have tested, and their job is to find out which one.
Ask students:
- How could you test the unknown powder so that you could identify it?
Students should realize that they will need to test the unknown powder the same way they tested all the other known powders and compare the results. If the unknown powder reacts with each test solution the same way one of the known powders did, then these two powders must be the same.
Question to Investigate
Can you use the characteristic ways substances react to identify an unknown powder?
Materials for each group
- Unknown in cup (The Unknown is baking powder)
- 1 Popsicle stick
- Testing sheet
- 4 test solutions
- 4 droppers
Procedure
- Place four samples of your group’s unknown powder in the “Unknown” column on the testing chart.
- Test the unknown with each test solution in the same way you tested each of the other powders.
- Compare the set of reactions for the unknown with those of the other powders.
Expected Results
The unknown will react with each test solution the same way that baking powder does because the unknown is baking powder.
4 Explain
Step 5
Have students report the identity of the unknown and discuss what evidence led them to their conclusion.
Tell students that they were able to use their observations to identify the unknown because each powder had its own set of characteristic chemical reactions with the test solutions.
Ask students:
- What is the identity of the unknown?
Baking powder is the unknown. - Which observations led you to your conclusion?
The unknown reacted with each test solution the same way baking powder did.
Explain that each substance is made up of certain molecules which interact with the molecules in each test liquid in a characteristic way. Some of these interactions result in a chemical reaction and others do not. However, each observation students made is based on the way the molecules of each powder interact with the molecules of each test solution.
5 Extend
Step 6
Have students identify the two substances in baking powder that make it bubble when water is added.
Remind students that baking powder was the only substance that bubbled when water was added to it. Bubbling in a chemical reaction is a sign that a gas is one of the products. Tell students that baking powder is a combination of different powders—baking soda, cream of tartar, and cornstarch. Two of these three react with one another and produce a gas when water is added. Students will need to test all the possible combinations of two powders with water. This way they can figure out which two powders cause baking powder to bubble with water.
Question to Investigate
Which two substances in baking powder react with one another and produce a gas when water is added?
Materials for Each Group
- Baking soda in a cup
- Cornstarch in a cup
- Cream of tartar in a cup
- 3 Popsicle sticks
- Toothpicks
- Wax paper
- Water
- Dropper
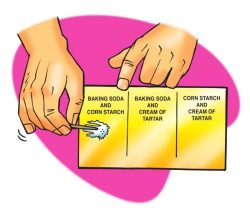
Procedure
- Use separate popsicle sticks to place a small amount of two powders on a piece of wax paper.
- Use a toothpick to mix the powders.
- Use a dropper to add about 5 drops of water to the combined powders and record your observations.
- Repeat steps 1 and 2 until you have tested all three combinations.
Expected results
The two combined powders that bubble with water are baking soda and cream of tartar.
Step 7
Demonstrate that vinegar and cream of tartar are both acids.
Point out that the mixture of baking soda and cream of tartar reacts with water to produce a gas. Baking soda and vinegar also react to produce a gas. Explain that carbon dioxide gas is produced in both reactions. Tell students that cream of tartar and vinegar have something else in common that they will investigate in the next demonstration.
Question to Investigate
Will vinegar turn universal indicator solution pink the way that cream of tartar does?
Materials for the Demonstration
- Universal indicator
- Cream of tartar
- Vinegar
- 2 clear plastic cups
- Popsicle stick
- Dropper
Teacher Preparation
- Use the indicator left over from the lesson.
- Use vinegar and cream of tartar left over from one of the student groups.

Procedure
- Pour about 25 mL of universal indicator solution in two separate clear plastic cups.
- Add 2 or 3 drops of vinegar to one cup.
- Scoop up a small amount of cream of tartar with the tip of a popsicle stick and add it to the other cup.
- Swirl both cups.
Expected Results
The indicator in both cups will turn pink.
Tell students that the color changes of indicator solution can tell you whether a substance is an acid or not. Because universal indicator turns pink when acids are added to it, we can say that both vinegar and cream of tartar are acids. When an acid reacts with baking soda, carbon dioxide gas is produced.
Ask students
- Why do you think the baking soda and cream of tartar reaction is similar to the baking soda and vinegar reaction?
Cream of tartar and vinegar are both acids and interact with sodium bicarbonate in a similar way to produce carbon dioxide gas.
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Simulations
No simulation available
Downloads
For Students
- Lesson 6.6 Student Activity Sheet PDF | DOCX | Google Doc
(Includes Testing Chart)
For Teachers
- Lesson 6.6 Lesson Plan PDF | DOCX | Google Doc
- Lesson 6.6 Activity Sheet Answers PDF | DOCX | Google Doc
Resources for the entire Chapter 6
- Chapter 6 Student Reading PDF | DOCX | Google Doc
- Chapter 6 Test Bank PDF | DOCX | Google Doc
Interactive Lesson Modules
- Lesson 6.6 Online Assignments Google Form
Have Questions? Visit Help Center
