Water is a Polar Molecule
Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts
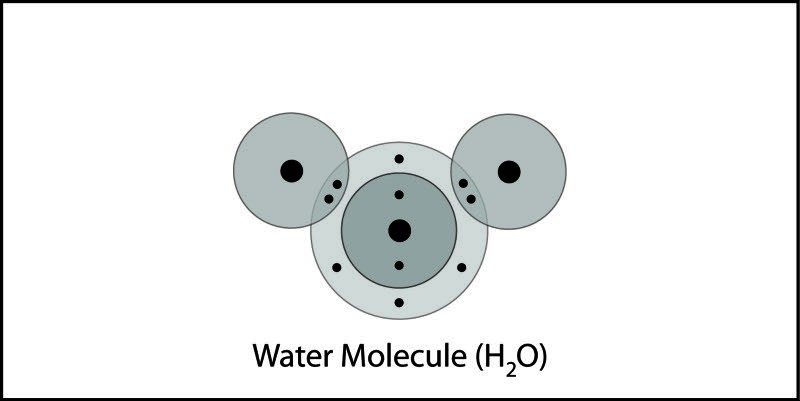
- The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral.
- In a water molecule, the oxygen atom and hydrogen atoms share electrons in covalent bonds, but the sharing is not equal.
- In the covalent bond between oxygen and hydrogen, the oxygen atom attracts electrons a bit more strongly than the hydrogen atoms.
- The unequal sharing of electrons gives the water molecule a slight negative charge near its oxygen atom and a slight positive charge near its hydrogen atoms.
- When a neutral molecule has a positive area at one end and a negative area at the other, it is a polar molecule.
- Water molecules attract one another based on the attraction between the positive end of one water molecule and the negative end of another.
Summary
Students will be introduced to the idea that water has a slight positive charge at one end of the molecule and a slight negative charge at the other (a polar molecule). Students view animations, make illustrations, and use their own water molecule models to develop an understanding of how the polar nature of water molecules can help explain some important characteristics of water
Objective
Students will be able to explain, on the molecular level, what makes water a polar molecule. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid.

Safety
Be sure you and the students wear properly fitting goggles. Isopropyl alcohol is flammable. Keep it away from flames or spark sources. Read and follow all warnings on the label. Use in well-ventilated room. Dispose of small amounts down the drain or according to local regulations. Have students wash hands after the activity.
Materials for Each Group
- Styrofoam water molecule models from Chapter 2, Lesson 2 (two per student)
- Permanent markers (blue and red)
- Isopropyl alcohol (70% or higher)
- Water
- Brown paper towel
- Droppers
Note About the Materials
Students made molecular models of the water molecule using Styrofoam balls and toothpicks in Chapter 2, Lesson 2. Give each student two of these water molecule models for this activity.
Download All Lesson 5.1 Resources
Get the entire lesson plan and Student Activity Sheet for "Lesson 5.1 - Water is a Polar Molecule."
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Instructions
1 Engage
Step 1
Show students examples of water molecules’ attraction for one another.
Remind students that in Chapters 1 and 2, they investigated the behavior of water at different temperatures and explored the state changes of water. Many of the explanations were based on the idea that water molecules are attracted to one another. Remind students that in Chapter 4 they looked at the covalent bonding between oxygen and hydrogen, which creates the water molecule. Now students will look more closely at the details of the covalent bonds in a water molecule to understand why water molecules are attracted to one another.
Project the video Water Balloon.
This video was shown in Chapter 1, Lesson 1 to show that water molecules are attracted to one another.
Project the video Water Fountain.
Point out that the water is able to stay together in these arcs because water molecules are very attracted to each other.
2 Evaluate
Give each student an activity sheet.
- Lesson 5.1 Student Activity Sheet PDF | DOCX | Google Doc
- Lesson 5.1 Activity Sheet Answers PDF | DOCX | Google Doc
Download the student activity sheet, and distribute one per student.
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms and Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
3 Explain
Step 2
Show molecular model animations that illustrate why water molecules are attracted to each other.
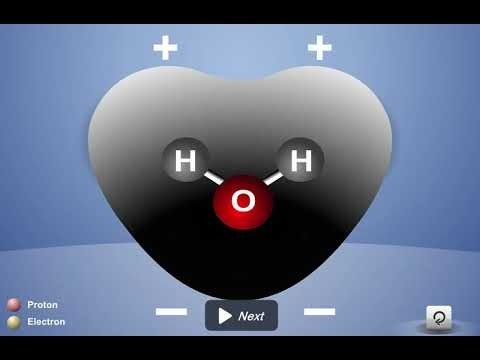
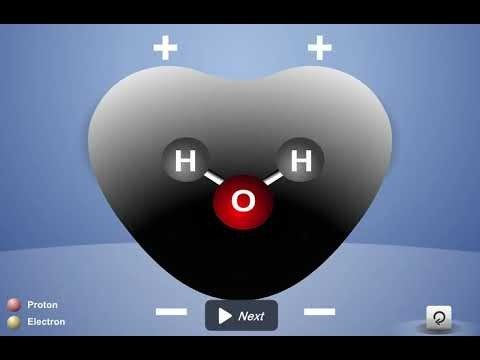
Project the animation Polar Water Molecule.
First Frame of the Animation:
Electrons are shared between atoms in a covalent bond.
Remind students how the shared electrons in a water molecule are attracted to the protons in both the oxygen and the hydrogen atoms. These attractions hold the atoms together.
Water molecules are neutral.
Be sure students realize that no protons or electrons are gained or lost. The water molecule has a total of 10 protons and 10 electrons (8 from the oxygen atom and 1 from each of the two hydrogen atoms). Since it has the same number of protons and electrons, the water molecule is neutral.
Click “Play”
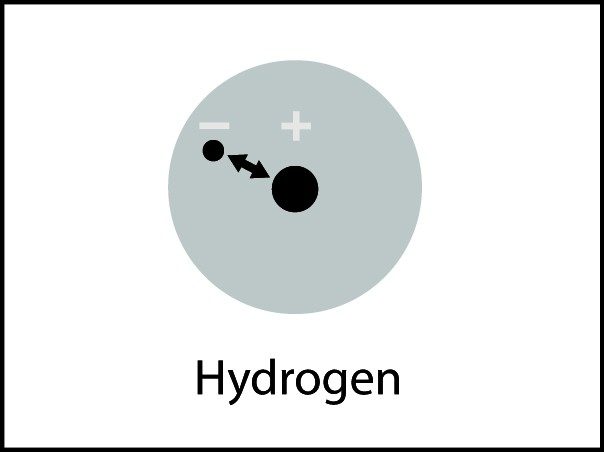
The electron cloud model shows where electrons are in a molecule.
Tell students that another way to see the difference in where the electrons are is by using the electron cloud model. Remind students that it’s impossible to know the exact location of an electron, so sometimes the regions occupied by electrons are shown as “clouds” around the nucleus in an atom or molecule.
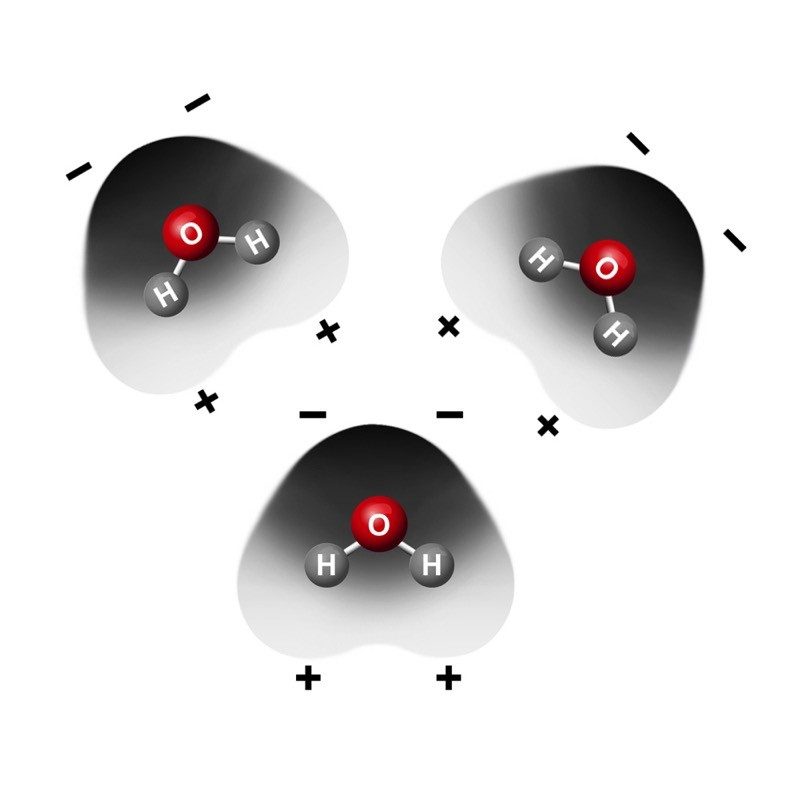
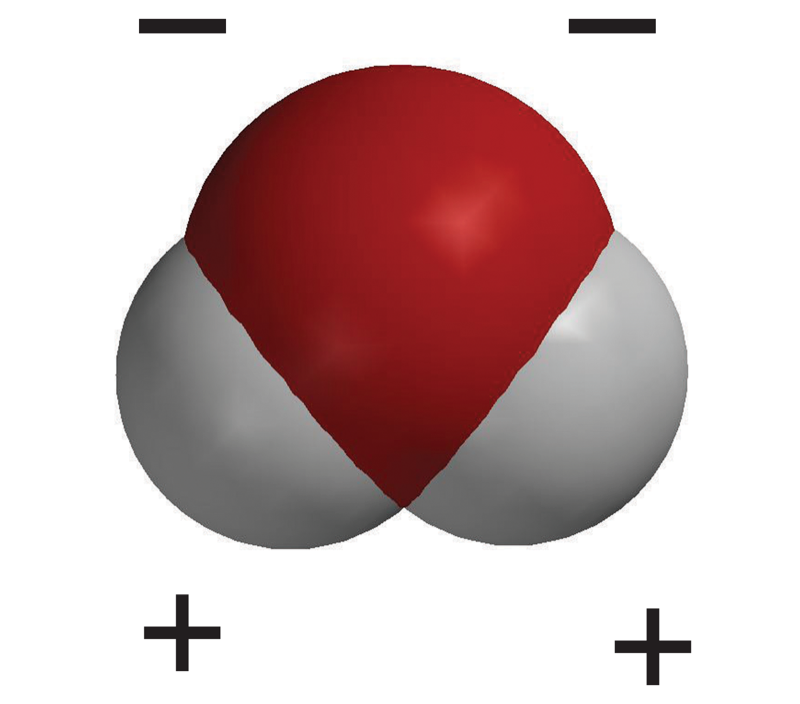
Unequal sharing of electrons makes water a polar molecule.
Tell students that the oxygen atom attracts electrons a little more strongly than hydrogen does. So even though the electrons from each atom are attracted by both the oxygen and the hydrogen, the electrons are a bit more attracted to the oxygen. This means that electrons spend a bit more time at the oxygen end of the molecule. This makes the oxygen end of the molecule slightly negative. Since the electrons are not near the hydrogen end as much, that end is slightly positive. When a covalently bonded molecule has more electrons in one area than another, it is called a polar molecule.
- The electron cloud model can show an unequal sharing of electrons.
Point out that the electron cloud around the oxygen is darker than the electron cloud around the hydrogen. This shows that electrons are more attracted to the oxygen end of the molecule than the hydrogen end, making the water molecule polar.
Click “Next”
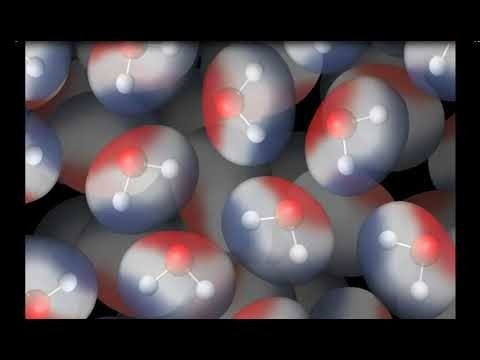
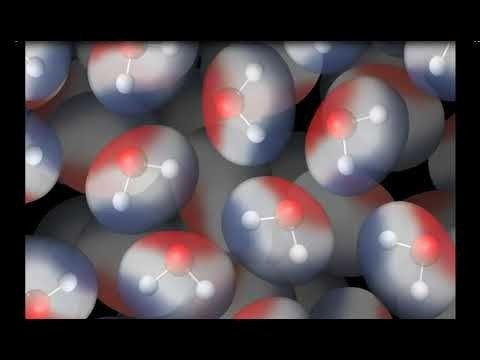
Color can be added to an electron cloud model to show where electrons are more or less likely to be.
Tell students that this is another model of a water molecule. In this model, color is used to show the polar areas of the water molecule. The negative area near the oxygen atom is red, and the positive area near the hydrogen atoms is blue.
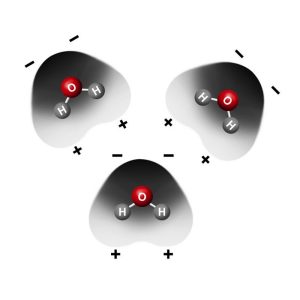
Project the animation Attraction Between Water Molecules.
Ask students:
What do you notice about the way water molecules orient themselves?
The red (oxygen) area of one water molecule is near the blue (hydrogen) end of another water molecule.
Why do water molecules attract one another like this?
Since the oxygen end of a water molecule is slightly negative and the hydrogen end is slightly positive, it makes sense that water molecules attract one another.
Step 3
Show students that the bonds between atoms in a molecule are different from the polar attractions between molecules.
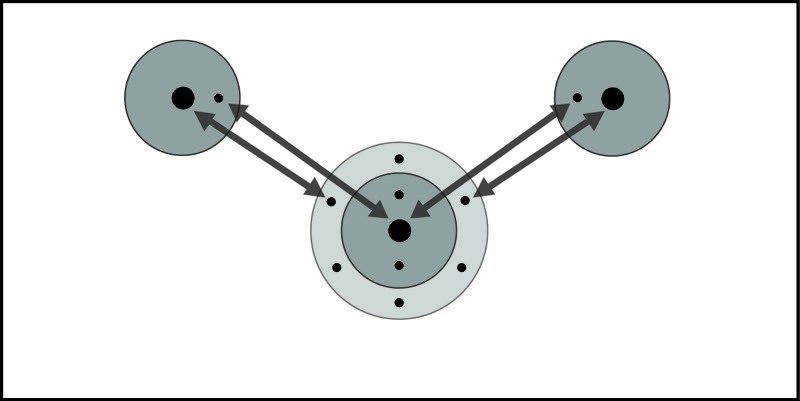
Project the image Attractions on Different Levels
Students may be confused about the bonds within a water molecule and the attractions between water molecules.
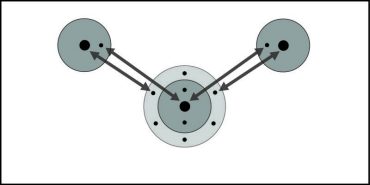
The bonds within molecules and the polar attractions between molecules
Explain to students that the interaction between the oxygen of one water molecule and the hydrogen of another is different than the sharing of electrons between the oxygen and the hydrogens within the water molecule itself.
It’s all about attractions between positive and negative.
Point out to students that attractions between positive and negative works on three different levels.
1. A single atom stays together because of the attraction between the positively charged protons and the negatively charged electrons.
2. In a molecule, two or more atoms stay together because of the mutual attraction between the positively charged protons from one atom and the negatively charged electrons from the other atom. This causes the covalent or ionic bonding that holds atoms or ions together.
3. Two or more water molecules stay together because of the positive and negative parts of the molecules attracting each other.
Step 4
Have students mark the positive and negative areas on a water molecule by color-coding their Styrofoam ball models.
Materials for Each Group
- Styrofoam water molecule models from Chapter 2, Lesson 2 (two per student)
- Permanent markers (blue and red)
Procedure
- Draw a blue “+” on each of the hydrogen atoms.
- Draw two red “–” at the bottom of the oxygen atom.
- Repeat this for your other water molecule.
- Position your water molecules so that opposite charges are near each other.
Ask students:
How do your Styrofoam ball models of water molecules relate to the color- coded charge density model shown in the animation?
The different colors show that water is a polar molecule.
What do the red “–” signs on the oxygen atom represent?
The red “–” signs represent the area where there are more electrons.
What do the blue “+” signs on the hydrogen atoms represent?
The blue “+” signs represent the area where there are fewer electrons.
Because water molecules are polar, how do they arrange themselves in liquid water?
The positive area of one water molecule is attracted to the negative area of another water molecule.
4 Explore
Step 5
Have students design a test to compare the rate of evaporation between water and alcohol.
Remind students that water molecules are very polar. The strong attractions between water molecules affect water’s surface tension, boiling point, and rate of evaporation. Tell students that they will do an experiment to compare the evaporation rates of water and another liquid that isn’t as polar.
Ask students:
Do you think a substance like water with polar molecules would evaporate faster or slower than a substance like alcohol with molecules that are not as polar?
The more-polar molecules will stick together more and will probably evaporate more slowly than less polar molecules. Less-polar molecules should evaporate faster because they are not as attracted to each other.
- How could you design a quick and easy evaporation test to compare the rate of evaporation between water and alcohol?
- What materials will you need?
- Should you use the same amount of water and alcohol?
- How will you know if one evaporates faster than the other?
- Is there a way to do it so that it will not take a lot of time?
Students should say that they will need the same small amount of water and alcohol. These liquids should be placed at the same time on a surface like a brown paper towel so that students can tell when each liquid evaporates.
Step 6
Have students follow the procedure below to compare the rate of evaporation between water and alcohol.
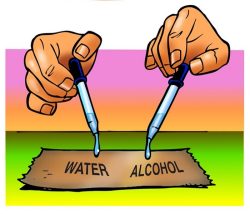
Question to Investigate
Does water evaporate faster or slower than less-polar alcohol?
Materials for Each Group
- Isopropyl alcohol (70% or higher)
- Water
- Brown paper towel
- Droppers
1. At the same time, place 1 drop of water and 1 drop of alcohol on a brown paper towel. Observe.
Expected Results
The dark spot on the paper towel made by the alcohol will turn lighter faster than the dark spot made by the water. This indicates that the alcohol evaporates more quickly than the water.
Note: This test is fine for middle school students but there is something about the test that does not make it completely fair. There are many more water molecules in a drop of water than alcohol molecules in a drop of alcohol. The test would be more fair if the same number of water and alcohol molecules are placed on the paper towel. This requires a way to “count” molecules. Determining the number of particles in a sample is a basic concept in chemistry, but is beyond the scope of a middle school chemistry unit. Even if the same number of water and alcohol molecules were used in this activity, the alcohol would evaporate faster.
5 Explain
Step 7
Discuss student observations and describe the differences in polarity between water and alcohol molecules.
Ask students:
Which evaporated faster, water or alcohol?
The alcohol evaporated faster.
Tell students that understanding about polarity can help explain why water evaporates more slowly than alcohol.
Project the image Water and Alcohol Molecules.
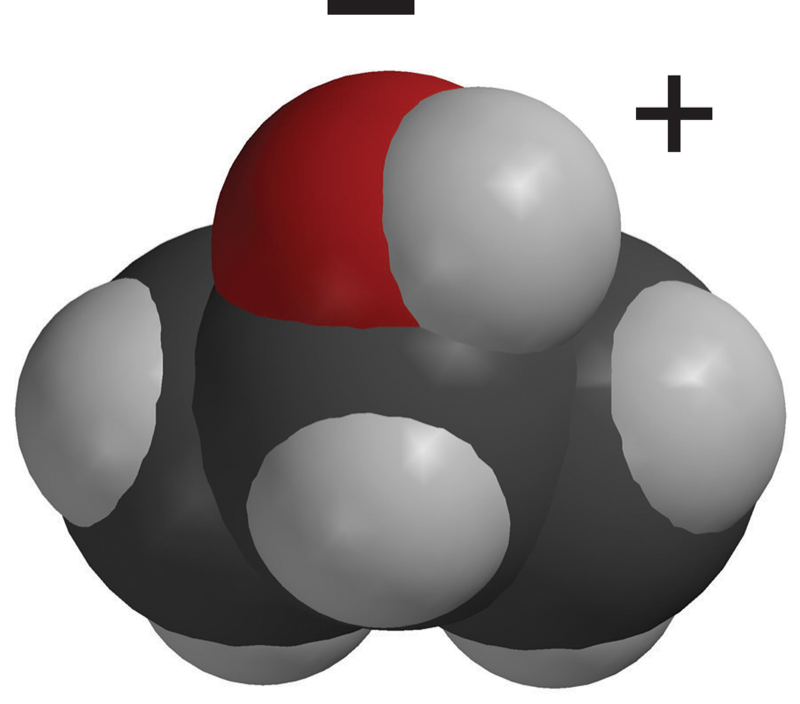
Remind students that the oxygen-hydrogen (O–H) bonds in water make it a polar molecule. This polarity makes water molecules attracted to each other.
Explain that the oxygen–hydrogen (O–H) bond in the alcohol molecule is also polar. But, the carbon–hydrogen (C–H) bonds in the rest of the alcohol molecule are nonpolar. In these bonds, the electrons are shared more or less evenly.
Because there are both polar and nonpolar areas on the alcohol molecule, they are somewhat less attracted to each other than water molecules are to each other. This makes it easier for alcohol molecules to come apart and move into the air as a gas. This is why alcohol evaporates faster than water.
6 Extend
Step 8
Have students consider how polarity might affect the temperature at which water and alcohol boil.
You know that water and alcohol have different characteristics because of the molecules they are made of and how these molecules interact with each other.
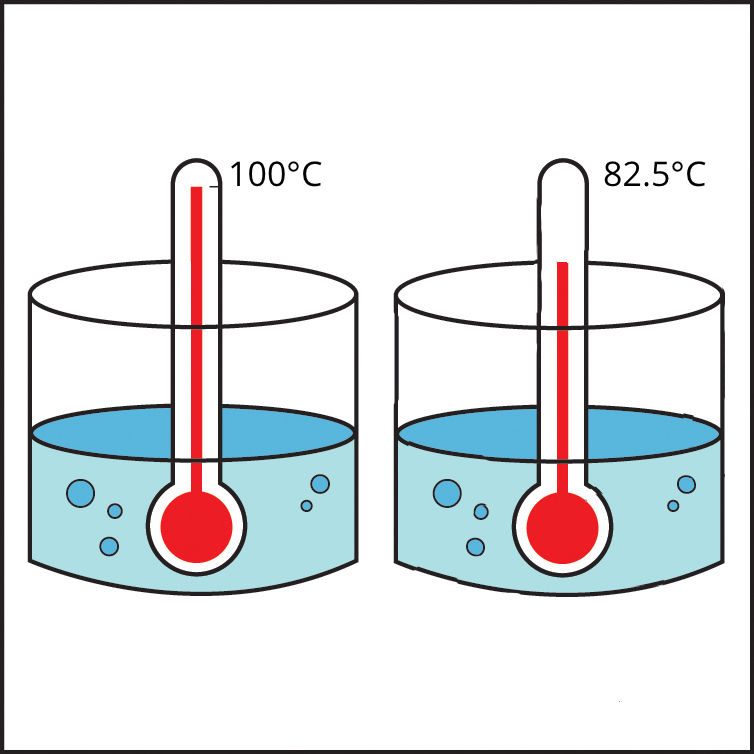
Project the image Water and Alcohol Boiling.
This illustration shows that alcohol boils at a lower temperature than water.
Water boils at 100 °C
Alcohol boils at 82.5 °C
Ask students:
Knowing what you do about the polarity of water and alcohol, explain why alcohol boils at a lower temperature than water.
The polar characteristic of water molecules causes them to attract each other well. The less polar alcohol molecules do not attract one another as strongly as water molecules do. It takes more energy to make water boil than it does to make alcohol boil. In other words, alcohol boils at a lower temperature than water.
Downloads
- Lesson 5.1 Student Activity Sheet PDF | DOCX | Google Doc
For Teachers
- Lesson 5.1 Lesson Plan PDF | DOCX | Google Doc
- Lesson 5.1 Activity Sheet Answers PDF | DOCX | Google Doc
- Lesson 5.1 Teacher Background PDF
Resources for the entire chapter 5
- chapter 5 Student Reading PDF | DOCX | Google Doc
- chapter 5 Test Bank PDF | DOCX | Google Doc
Interactive Lesson Modules
- Lesson 5.1 Online Assignments Google Form
Have Questions? Visit Help Center