Lesson 5.1: Water is a Polar Molecule
Accompanying Lesson Plan: Lesson 5.1: Water is a Polar Molecule
Video
Water Balloon
- Water molecules are so attracted to each other that even after the balloon pops, the water molecules stay together keeping the shape of the balloon.
Video
Water Fountain
- Water stays together because water molecules are attracted to one another.
Interactive
Polar Water Molecule
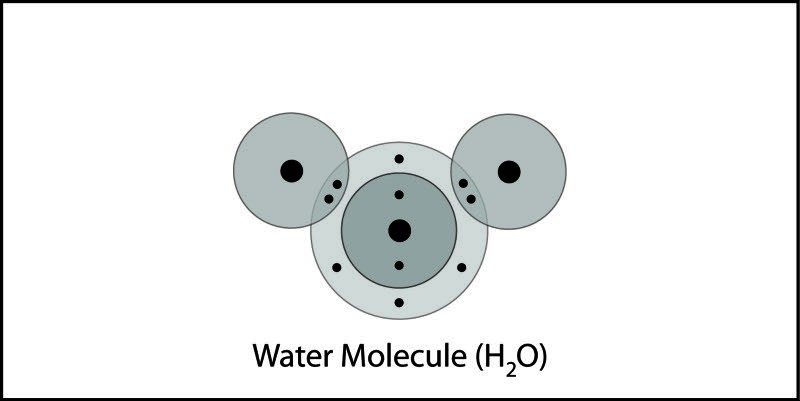
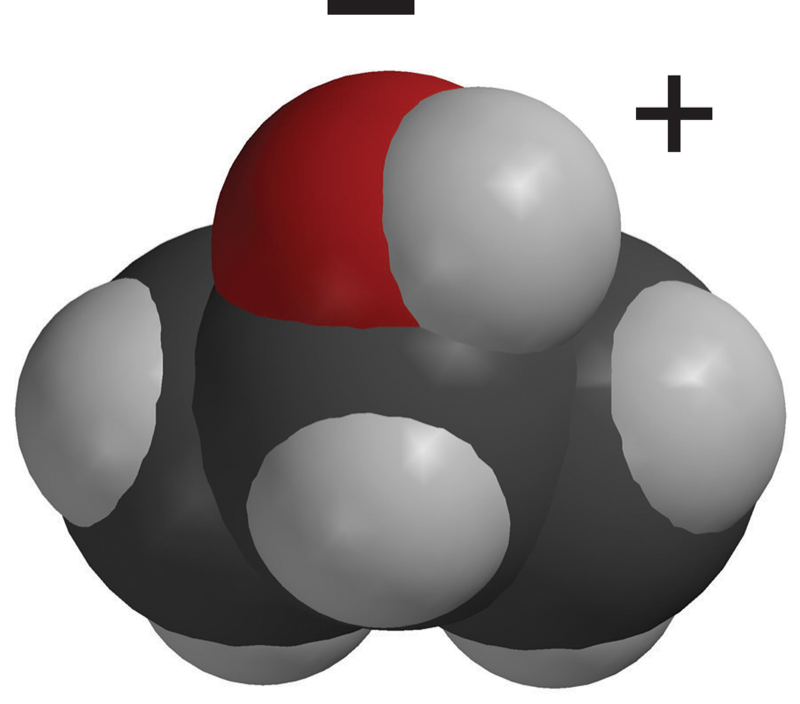
- In the water molecule, the oxygen and hydrogen atoms share electrons in covalent bonds.
- There are a total of 10 protons and 10 electrons, so the water molecule is neutral.
- The electron cloud model shows that electrons are not shared equally in the water molecule.
- Electrons are a bit more attracted to the oxygen atom than they are to the hydrogen atoms.
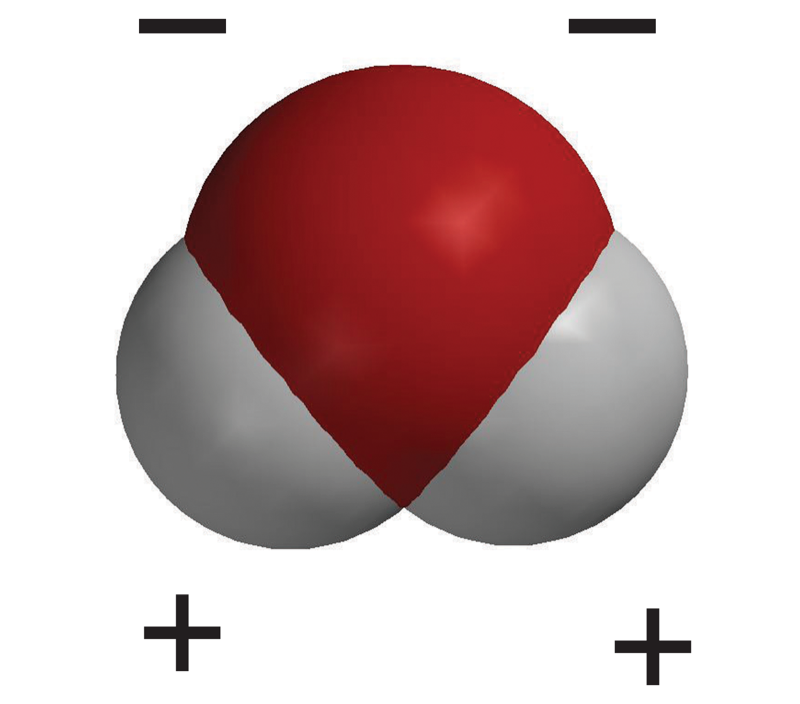
- This makes the water molecule slightly negative at the oxygen end and slightly positive at the hydrogen end.
Video
Attraction Between Water Molecules
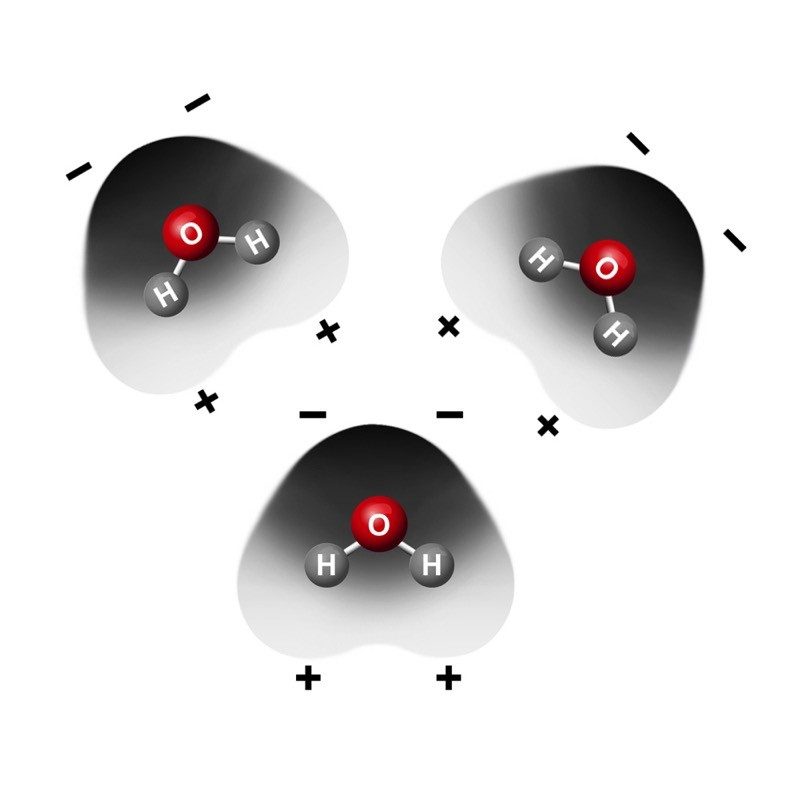
- Water molecules tend to orient themselves with the positive area of one molecule near the negative area of another.
Image
Attractions on Different Levels
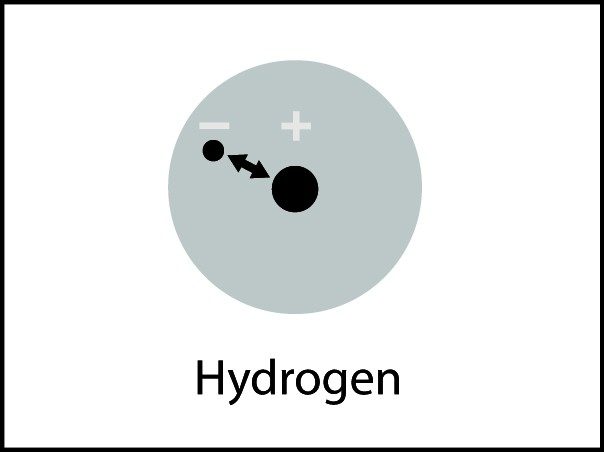
- Electrons are attracted to protons within an atom. This is what keeps the atom together.
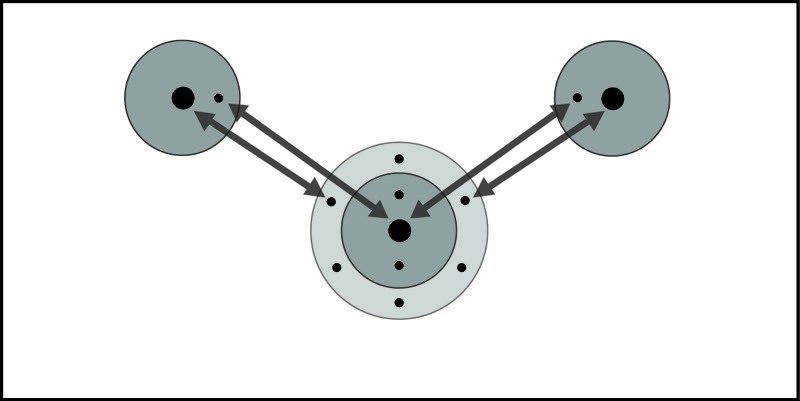
- Electrons are attracted to the protons of other atoms. This is what causes atoms to bond and what holds a molecule together.
- Electrons can be shared unequally in a molecule, creating a polar molecule. Opposite polar ends of molecules attract and hold one molecule to another.