Carbon Dioxide Can Make a Solution Acidic
Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts
Carbon dioxide (CO2) gas dissolved in water can cause water to become acidic.
The acidity of water from dissolved CO2 can be reduced by a base such as baking soda (sodium bicarbonate).
Summary
The teacher blows into a universal indicator solution until it changes color. Students interpret this color change and explain that the solution becomes acidic. Students explore whether carbon dioxide from other sources, namely carbonated water and a chemical reaction between baking soda and vinegar, can also make a solution acidic. Students then apply their observations to the environmental problem of ocean acidification by doing research on this issue.
Objective
Students will be able to explain that carbon dioxide from any source reacts chemically with water to form carbonic acid. They will also be able to use the color changes of universal indicator to monitor the changing pH of a solution during a chemical reaction.

Safety
Be sure you and the students wear properly fitting goggles during the activity and wash hands afterwards. Universal indicator is alcohol-based and flammable. Use vinegar in a well-ventilated room. Read and follow all safety warnings on the label. Dispose of all liquid waste down the drain or according to local regulations.
Materials for Demonstration
- Universal indicator solution
- Water
- 2 clear plastic cups
- Straw
Materials for Each Group
- Water
- Universal indicator solution in cup
- Universal indicator pH color chart
- Carbonated water (club soda or seltzer water) in wide, clear, plastic cup
- Baking soda in wide, clear, plastic cup
- Vinegar
- Alka-Seltzer tablet
- 2 small clear plastic cups
- 4 wide clear plastic cups
- 4 taller, clear, plastic cups
- Graduated cylinder
- Snack-sized zip-closing plastic bag
About the Materials
For this lesson, each group will need a Universal Indicator pH color chart. Print enough pages of these charts on a color printer so that each group can have their own chart.
- Universal Indicator pH Color Chart PDF
Download All Lesson 6.10 Resources
Get the entire lesson plan and Student Activity Sheet for "Lesson 6.10 - Carbon Dioxide Can Make a Solution Acidic."
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Instructions
1 Engage
Step 1
Do a demonstration to show that adding CO2 gas to water can make the water become acidic.
Materials for the Demonstration
- Universal indicator solution
- Water
- 2 clear plastic cups
- Straw
Teacher Preparation
Make indicator solution for student groups
- Make a dilute universal indicator solution for this demonstration and for each student group by combining 625 mL water with 25 mL universal indicator solution.
- Pour at least 80 mL of this dilute universal indicator solution into a clean plastic cup for each student group.
Note: Your local tap water is likely fine for the demonstration and activities in this lesson. If the indicator solution you make is not green, this means that your water is either acidic or basic. If this happens, use distilled water, which is available in supermarkets and pharmacies.
Note: In the activities, each group will need 80 mL of indicator solution. Check to make sure that you prepare enough solution. You will need about 50–60 mL of indicator solution for your demonstration. If 650 mL of solution is not enough, make more using the same proportions.

Procedure
- Show students both samples of universal indicator solution. Place a straw in one of the samples so that the straw goes all the way to the bottom of the cup.
- Hold the cup so that students can clearly see the liquid. Blow into the straw until the indicator solution changes from green to yellow.
Ask students:
- Does blowing into the indicator solution change its pH?
Yes, the color changes, so there must be a change in pH, too. - Does the solution become a little more acidic or a little more basic?
The color change shows that the solution is a little more acidic.
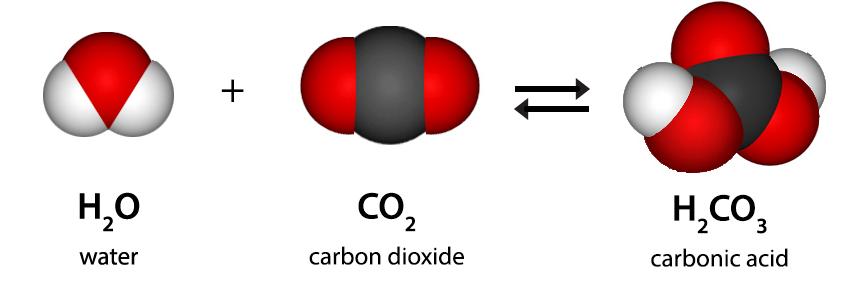
Tell students that a chemical reaction occurs between the molecules of CO2 and the molecules of H2O to create a very small amount of an acid called carbonic acid (H2CO3).
2 Evaluate
Give each student an activity sheet.
- Lesson 6.10 Student Activity Sheet PDF | DOCX | Google Doc
- Lesson 6.10 Activity Sheet Answers PDF | DOCX | Google Doc
Download the student activity sheet, and distribute one per student.
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms & Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
3 Explore
Step 2
Have students use club soda as a source of CO2 to see if the gas will change the pH of an indicator solution.
Question to Investigate
Will carbon dioxide from carbonated water change the pH of an indicator solution?
Materials for Each Group
- Universal indicator solution in a plastic cup
- Water
- Carbonated water (club soda or seltzer water) in a wide clear plastic cup
- 1 wide, clear, plastic cup
- 2 taller, clear, plastic cups
- Graduated cylinder
- Universal Indicator pH Color Chart
Teacher Preparation
Pour 25 mL of carbonated water into a wide, clear, plastic cup for each group.
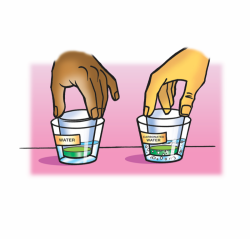
Procedure
- Measure 30 mL of universal indicator solution and divide it evenly into two small, clear, plastic cups.
- Add 25 mL of water to a wide plastic cup and 25 mL of carbonated water to another wide cup.
- Stand the small cups with indicator solution in the liquid in the wider cups as shown.
- Turn the two tall cups upside down and place them over the two wider cups.

- While holding the top and bottom cups to keep them together, gently swirl both sets of cups. Watch the color of the indicator in both cups to see if there is any change.
- Compare the color of the indicator to the pH Color Chart to find out whether the solution is acidic, neutral, or basic.
Expected Results
The indicator inside the cups with water remained green, while the indicator with the carbonated water turned yellow.
Step 3
Discuss student observations and what will happen in the following activity.
Ask students:
- Did either indicator change color?
Only the indicator with the carbonated water changed color. - What does the color change tell you about the pH of the indicator solution? Is it acidic or basic?
The indicator solution is now acidic. - The carbonated water should not have splashed into the indicator. Why did the indicator solution change color in one set of cups?
The carbon dioxide from the carbonated water dissolved in the indicator solution. The molecules of carbon dioxide reacted with the water, forming carbonic acid, and changed the color of the indicator.
Tell students that they have seen carbon dioxide gas from your breath and carbon dioxide gas from carbonated water turn an indicator solution acidic.
Ask students:
- Do you think carbon dioxide gas produced during a chemical reaction will also turn an indicator solution acidic?
Carbon dioxide from any source should cause the indicator solution to become acidic. The amount of carbon dioxide gas produced and dissolved in the indicator solution may cause the color of the indicator to vary, but on the acidic side. - What chemical reaction do you know of that can produce carbon dioxide gas?
Students should remember that vinegar and baking soda react, producing carbon dioxide gas. Tell students that they will combine baking soda and vinegar in the next activity.
Step 4
Use a chemical reaction to produce CO2 to see if it changes the pH of an indicator solution.
Question to Investigate
Will carbon dioxide gas produced in the baking soda and vinegar reaction change the pH of an indicator solution?
Materials for Each Group
- Universal indicator solution in cup
- Universal indicator pH color chart
- Water
- Baking soda in wide clear plastic cup
- Vinegar in cup
- 2 small clear plastic cups
- 1 wide clear plastic cups
- 2 taller clear plastic cups
- Graduated cylinder
Teacher Preparation
- Pour about 50 mL of vinegar in a wide plastic cup for each group.
- Place about ½ teaspoon of baking soda into a small clear plastic cup for each group.
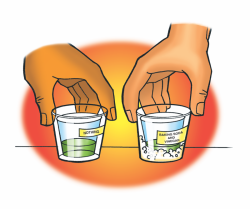
Procedure
- Measure and pour 25ml of vinegar into two wide plastic cups.
- Pour 15ml of universal indicator into two clean small plastic cups.
- Pour all the baking soda into one of the cups of vinegar. Do not pour anything into the other.
- Stand the small cups with indicator solution in both the wider cups as shown.

- Turn the two tall cups upside down and place them over the two wider cups.
- While holding the top and bottom cups to keep them together, gently swirl both sets of cups. Watch the color of the indicator in both cups to see if there is any change.
- Compare the color of the indicator to the pH Color Chart to find out whether the solution is acidic, neutral, or basic.
Expected Results
The indicator inside the cup with only vinegar remained green while the indicator inside the cup with the vinegar and baking soda reaction turned yellow.
Step 5
Discuss student observations.
Ask students:
- Did either indicator change color?
Only the indicator with the chemical reaction changed color. - Why did one set of cups only have vinegar in the bottom?
It is possible that vinegar by itself causes the indicator to change color. Since this indicator did not change color, it must be the carbon dioxide gas produced by the chemical reaction, and not just the vinegar that caused the color change. The indicator solution in the set of cups with only vinegar in the bottom serves as a control. - What does the color of the indicator solution tell you about the pH of each solution? Is it acidic, neutral, or basic?
The color change shows that the indicator solution is slightly acidic. - What could you add to the acidic indicator solution to neutralize it?
Because the indicator solution is acidic, students should suggest adding a base. Tell students that baking soda is a base.
4 Explain
Step 6
Explain that carbon dioxide from any source can make water acidic.
Ask students:
- What did carbon dioxide from breath, carbonated water, and the baking soda and vinegar reaction all do to water?
The CO2 from each source reacted with the water and made it acidic.
Project the illustration CO2 Reacting with Water.
Tell students that carbon dioxide reacts with water to produce carbonic acid. Students may count the number of atoms on each side of the equation to show that it is balanced. Point out that the double arrow in this equation means that carbonic acid breaks down readily to form carbon dioxide and water again.
Explain to students that too much CO2 in the atmosphere causes Earth and its atmosphere to become warmer. But excess CO2 can do something else which they have seen in the chemical equation and in their experiments. Carbon dioxide can make water more acidic which is causing a big problem in the oceans. The excess acid in ocean water, called ocean acidification, makes it difficult for some organisms to form shells and is especially damaging to coral.
5 Extend
Step 7
Explain how ocean acidification is bad for shell-making organisms and show a video about ocean acidification.
Explain that the ocean is actually basic. The pH of the ocean is about 8.2. The term “ocean acidification” means that the ocean is tending to become more acidic or less basic. It has moved from about 8.2 to 8.1. This may not seem like a big change, but it is a very big change to organisms in the ocean which are very sensitive to changes in pH. When ocean water becomes more acidic it causes two main problems for shell-making organisms like clams, oysters, and coral:
- It becomes harder for these organisms to make their shells
- If the water becomes too acidic, normal shells can react with the more acidic water causing the shell to break down
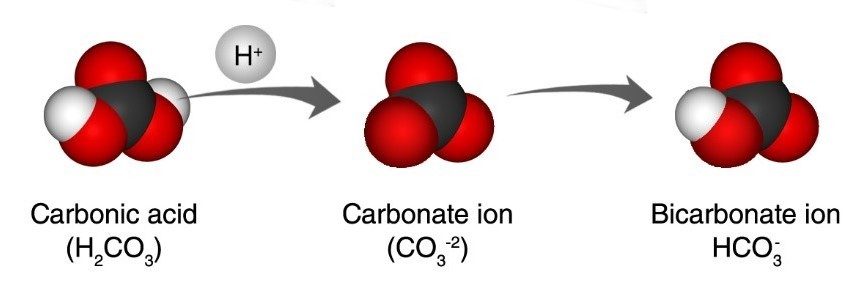
Clams, oysters, coral and other shell-making organisms make their shells out of two ions: the calcium ion (Ca+2) and the carbonate ion (CO-2). When these two ions join, they make calcium carbonate (CaCO3) which is the main substance for the structure of the shell. Ocean acidification affects the carbonate ion. Here’s how:
The site also offers a wealth of resources on ocean acidification and climate change.
Project the illustration Carbonic acid and Carbonate ion.
Remind students that water and carbon dioxide react to form carbonic acid.
A hydrogen atom from the carbonic acid gets into the water as a hydrogen ion (H+). This hydrogen ion bonds to the carbonate ion in ocean water and creates bicarbonate ion (HCO-3) which the shell-making organisms can’t use. This means there are fewer carbonate ions for the creatures to attach a calcium ion to, making it harder for them to make the calcium carbonate they need to make their shells.
Extra hydrogen ions in the water also makes the water more acidic. If the water eventually gets too acidic it might react with the calcium carbonate in the shells causing them to break down.
Show the video Ocean Acidification.
Note: The narration and action of the video go by pretty quickly so you might want to stop the video in a few places to help students understand what is being presented.
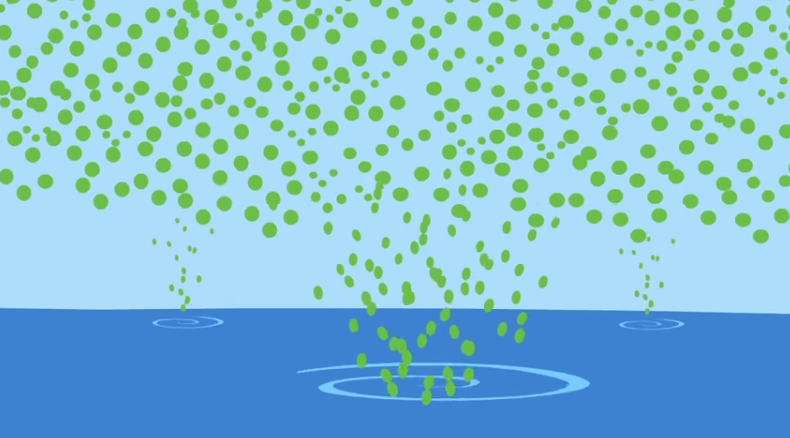
The green dots represent excess carbon dioxide in the atmosphere due to the burning of fossil fuels. The ocean absorbs a large amount of this carbon dioxide.

The little orange characters represent carbonate ions which the shell-making organisms need. They use carbonate ions and calcium ions to make calcium carbonate to build their shells.

Carbon dioxide reacts with water and produces carbonic acid (green irregular blob) which produces hydrogen ions. These ions bond to the carbonate ions and create a substance (bicarbonate ion not shown) that the organisms can’t use.

With shells difficult to make, clams and other shell-making organisms will be smaller and not reproduce as much so the creatures that eat them may not get enough food. This could affect the whole food chain.

The extra hydrogen ions don’t just bond to the carbonate ion, they also make the water more acidic.

The oceans could become so acidic in the future that the calcium carbonate shells could react with the water and break down.
Step 8
Have students do research on ways to reduce the amount of carbon dioxide released into the atmosphere.
The vast majority of the excess carbon dioxide in Earth’s atmosphere is from burning fossil fuels such as petroleum, natural gas, and coal. Most of this fuel is used for cars, trucks and other forms of transportation, for running power plants that produce electricity, and for heating homes and businesses.
Have students research alternative sources of energy that could help in burning less fossil fuel. Students could present their research in a short paper with illustrations, power point, tri-fold board, or in any way you feel will work. Students should describe how the renewable energy source works and the advantages and challenges of the technology.
Some possible topics could be:
Renewable energy sources
- Wind
- Solar
- Geothermal
- Biofuels
- Hydroelectric
New transportation technology
- Electric cars
- Hydrogen fuel cells
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Downloads
For Students
- Lesson 6.10 Student Activity Sheet PDF | DOCX | Google Doc
For Teachers
- Lesson 6.10 Lesson Plan PDF | DOCX | Google Doc
- Lesson 6.10 Activity Sheet Answers PDF | DOCX | Google Doc
Resources for the entire Chapter 6
- Chapter 6 Student Reading PDF | DOCX | Google Doc
- Chapter 6 Test Bank PDF | DOCX | Google Doc
Interactive Lesson Modules
- Lesson 6.10 Online Assignments Google Form
Have Questions? Visit Help Center