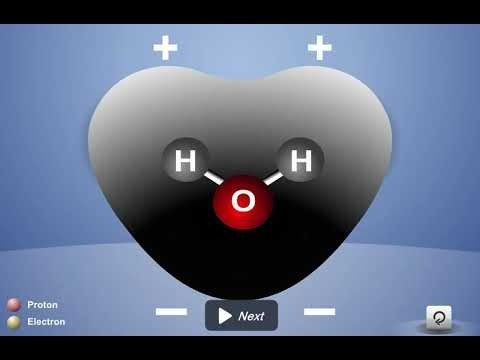
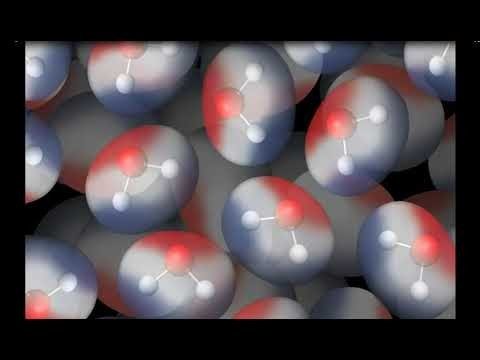
The Water Molecule and Dissolving
Students investigate the polarity of the water molecule and design tests to compare water to less polar liquids for evaporation rate, surface tension, and ability to dissolve certain substances. Students also discover that dissolving applies to solids, liquids, and gases.
Lessons:
- 5.1: Water is a Polar Molecule
- 5.2: Surface Tension
- 5.3: Why Does Water Dissolve Salt?
- 5.4: Why Does Water Dissolve Sugar?
- 5.5: Using Dissolving to Identify an Unknown
- 5.6: Does Temperature Affect Dissolving?
- 5.7: Can Liquids Dissolve in Water?
- 5.8: Can Gases Dissolve in Water?
- 5.9: Temperature Changes in Dissolving
Downloads
Chapter 5
Student Reading
A summary of the investigations and science content from all lessons in the chapter
Teacher Background
At any point while using a lesson, you could familiarize yourself with the following content background.
Reading Materials
- Lesson 5.1 Teacher Background (PDF):
- Counting Molecules
- Lesson 5.3 Teacher Background (PDF):
- Polarity
- Lesson 5.9 Teacher Background (PDF):
- Making and Breaking Bonds
- Counting Molecules