Lesson 5.2: Surface Tension
Accompanying Lesson Plan: Lesson 5.2: Surface Tension
Video
Water’s Surface Tension
- The paper clip is on the water but is not floating like a boat which is less dense than water.
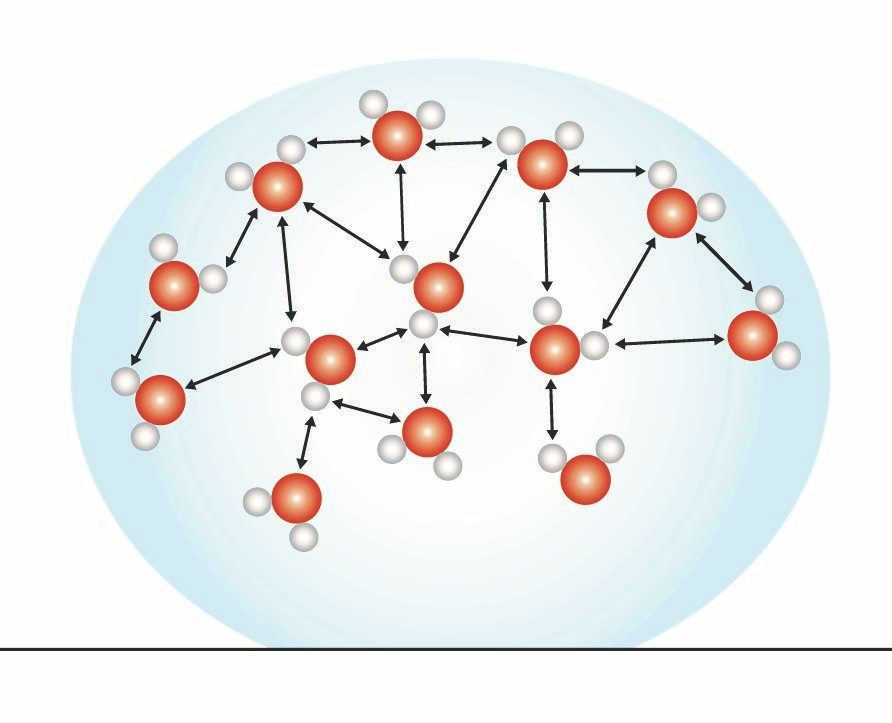
- The paper clip is more dense than water but can rest on the surface of the water because of the water's surface tension.
Video
Water on Paper Towel
- Paper is made from cellulose which is made of glucose molecules bonded together.
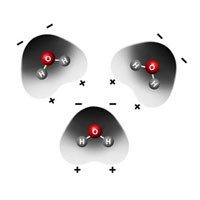
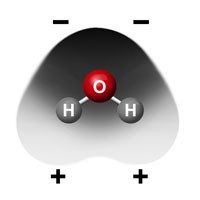
- Glucose has many O–H bonds which are polar.
- Polar water molecules are attracted to the polar cellulose and cling to it.
Video
Water on Wax Paper
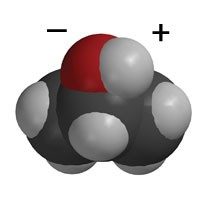
- Wax is made of paraffin which is only carbon-hydrogen bonds.
- C–H bonds are not polar.
- Water is more attracted to itself than to the wax so it stays together and does not spread out or absorb into the wax paper.