Neutralizing Acids and Bases
Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts
- pH is a measure of the concentration of H3O+ ions in a solution.
- Adding an acid increases the concentration of H3O+ ions in the solution.
- Adding a base decreases the concentration of H3O+ ions in the solution.
- An acid and a base are like chemical opposites.
- If a base is added to an acidic solution, the solution becomes less acidic and moves toward the middle of the pH scale. This is called neutralizing the acid.
- If an acid is added to a basic solution, the solution becomes less basic and moves toward the middle of the pH scale. This is called neutralizing the base.
Summary
Students will use citric acid and sodium carbonate solutions to see that adding a base to an acidic solution makes the solution less acidic. Students will then use a base to help them identify which of two acidic solutions is more concentrated.
Objective
Students will be able to explain, on the molecular level, that pH is affected by the concentration of the H3O+ ions in water. They will also be able to explain why adding a base to an acid or an acid to a base can make the pH of the solution closer to 7.

Safety
Be sure you and the students wear properly fitting goggles during the activity and wash hands afterwards. Sodium carbonate may irritate skin. Citric acid is an eye irritant. Universal indicator is alcohol-based and flammable. Read and follow all safety warnings on the label. At the end of the lesson, have students pour their used solutions in a waste container. Dispose of this waste down the drain or according to local regulations. The leftover citric acid and sodium carbonate powders can be disposed of with the classroom trash.
Materials for the Demonstration
- 4 clear plastic cups
- Graduated cylinder
- Universal indicator solution
- Water
- Sodium carbonate
- Citric acid
- Flat toothpicks
- 2 droppers
- Masking tape and pen or permanent marker
Materials for Each Group
- Universal indicator solution in cup
- Citric acid in cup
- Sodium carbonate in cup
- Water
- Solution A, sodium carbonate solution
- Solution B, more concentrated sodium carbonate solution
- At least 8 flat toothpicks
- Graduated cylinder
- Spot plate
- 4 droppers
- 3 clear plastic cups
- Masking tape and pen or permanent marker
About the Materials
Each group will need Universal Indicator Solution, citric acid (anhydrous), and sodium carbonate (anhydrous—Laboratory grade). Each group will also need a 6-well spot plate or a 12-well spot plate.
Download All Lesson 6.9 Resources
Get the entire lesson plan and Student Activity Sheet for "Lesson 6.9 - Neutralizing Acids and Bases."
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Instructions
1 Engage
Step 1
Do a demonstration to show students that an acidic solution becomes less acidic when drops of a base are added.
Materials for the Demonstration
- 4 clear plastic cups
- Graduated cylinder
- Universal indicator
- Water
- Sodium carbonate
- Citric acid
- Flat toothpicks
- 2 droppers
- Masking tape and pen or permanent marker
Teacher Preparation
Make indicator solution for student groups
- Make a dilute universal indicator solution for this demonstration and for each student group by combining 125 mL water with 5 mL universal indicator solution.
- Pour about 15 mL of this dilute universal indicator solution into a clean cup for each student group.
Note: Your local tap water is likely fine for the demonstration and activities in this lesson. If the indicator solution you make is not green, this means that your water is either acidic or basic. If this happens, use distilled water, which is available in supermarkets and pharmacies.
Note: In the engage and extend activities, students will fill 6 wells with universal indicator solution. Check to make sure that 15 mL of solution is enough. You will need about 25 mL of indicator solution for your demonstration. If 125 mL of solution is not enough, make more using the same proportions.
Prepare for the Demonstration
- Divide the remaining indicator solution into two clear plastic cups for you to use in the demonstration.
- Use masking tape and a pen to label two empty cups citric acid and sodium carbonate.
- Use your graduated cylinder to add 5 mL of water to each labeled cup.
- Use a flat toothpick to pick up as much citric acid as you can on the end of the toothpick as shown. Add this citric acid to the water in the citric acid cup. Gently swirl until the citric acid dissolves.
- Use a flat toothpick to pick up as much sodium carbonate as you can on the end of a toothpick. Add this sodium carbonate to the water in the sodium carbonate cup. Gently swirl until the sodium carbonate dissolves.
Procedure
- Hold up the two cups of universal indicator solution, which are both green.
- Also show students that you have a citric acid solution and a sodium carbonate solution.
Ask students:
- What color will the green indicator solution turn if I add a few drops of citric acid solution?
The indicator solution will change color toward red.
Procedure
- Add 3–5 drops of citric acid solution to one of the cups.
Expected Results
The color of the solution should change from green to reddish.
Ask students:
- What do you think you could add to the reddish indicator to make it less acidic and go back toward green?
Students should suggest adding sodium carbonate (a base) to the acidic (red) solution.

Procedure
- While holding up the cup of reddish indicator solution, add 1 drop of sodium carbonate solution, swirl, and compare the color of the solution to the color of the control.
- Add another drop if necessary to get closer to the green color of the control. Continue adding drops until the color gets close to green. If you add a drop and the color goes past green to blue, ask students what the blue color tells you about the solution. The blue indicates that the solution has gone from being acidic to basic.
Explain that acids and bases are like chemical opposites. Tell students that they will experiment to figure out how many drops of a basic solution it takes to cause an acidic solution to move to the middle of the pH scale. This is called neutralizing the acid.
2 Evaluate
Give each student an activity sheet.
- Lesson 6.9 Student Activity Sheet PDF | DOCX | Google Doc
- Lesson 6.9 Activity Sheet Answers PDF | DOCX | Google Doc
Download the student activity sheet, and distribute one per student.
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms & Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
3 Explore
Step 2
Have students prepare the solutions for the activity.
Teacher Preparation
Students will need small amounts of sodium carbonate and citric acid for the activity.
- Label two small plastic cups citric acid solution and sodium carbonate solution for each group.
- Place about ¼ teaspoon of citric acid and sodium carbonate in the labeled cups.
- Distribute the cups with universal indicator solution to each student group.
Materials for Each Group
- Sodium carbonate in cup
- Citric acid in cup
- Universal indicator in cup
- Water
- 3 clear plastic cups
- Graduated cylinder
- Flat toothpicks
- 2 droppers
- Spot plate
- Masking tape and pen or permanent marker

Procedure
Label your equipment
- Use masking tape and a pen to label one cup citric acid solution and another cup sodium carbonate solution.
- Use a small piece of masking tape and a pen to label one dropper citric acid solution and the other dropper sodium carbonate solution.

Make a citric acid solution
- Use your graduated cylinder to add 5 mL of water to the cup labeled citric acid.
- Use a flat toothpick to pick up as much citric acid as you can on the end of the toothpick as shown.
- Add this citric acid to the water in the citric acid cup. Gently swirl until the citric acid dissolves.

Make a sodium carbonate solution
- Use your graduated cylinder to add 5 mL of water to the cup labeled sodium carbonate.
- Use a flat toothpick to pick up as much sodium carbonate as you can on the end of a toothpick.
- Add this sodium carbonate to the water in the sodium carbonate cup. Gently swirl until the sodium carbonate dissolves.
Step 3
Have students neutralize an acidic solution.
Question to Investigate
How many drops of sodium carbonate solution will it take to neutralize your citric acid solution?
Materials for Each Group
- Universal indicator solution
- Citric acid solution
- Sodium carbonate solution
- At least 6 flat toothpicks
- Spot plate
- 3 droppers
Procedure
- Use a dropper to nearly fill two small wells in your spot plate with universal indicator solution. Do not add anything else to the first well. This will be your control.
- Add 3 drops of citric acid solution to the indicator in one of the wells. Use a clean toothpick to mix the solution. If it is not reddish, add more drops, but be sure to count the total number of drops added.
Ask students:
- What could you then add in order to make the indicator solution less acidic?
Adding a base, like the sodium carbonate solution, will make the solution less acidic. - Should you add one drop of sodium carbonate solution at a time or a few drops at once?
You should add one drop at a time to better monitor how many more drops of the sodium carbonate solution should be added. - How will you know when the solution is neutralized?
The color of the solution will be similar to the color of the control. Tell students that if the solution turns blue, it has gone from an acid, past neutral, and is now a base. If this happens, try adding one or more drops of citric acid until the color is close to neutral. Be sure to keep track of the total number of drops of acid and base you add.
- Add single drops of sodium carbonate to the same well in which you added the acid. Be sure to count the drops you use and stir with a toothpick after adding each drop.
Expected Results
With each drop of sodium carbonate, the citric acid solution will move toward neutral, eventually becoming green.
Note: The solution may get close to the green color of the control but will probably not be exact. This is because the citric acid and sodium carbonate solutions are not exactly equal in the way they act as acid and base. Also, to be very exact, students would need to be able to use half-drops or even quarter-drops, which is not possible with the droppers the students are using. As long as students see a trend toward the green control color, that is good enough.
How many drops of sodium carbonate does it take to neutralize your citric acid solution? | ||
Acidic solution |
Number of drops of citric acid solution added to the indicator | Number of drops of sodium carbonate solution needed to neutralize the citric acid solution |
First citric acid solution | 3 drops |
|
More concentrated citric acid solution |
|
|
Step 4
Discuss student observations.
- How many drops of sodium carbonate did it take to bring the color back to the color of the control?
Results will vary but it should take fewer drops of sodium carbonate than drops of citric acid to neutralize the solution. - Does the solution become more acidic or less acidic as each drop of sodium carbonate is added to the indicator?
The solution becomes less acidic. - How do you use the color of the control to help you neutralize an acid?
When the color of the universal indicator solution becomes near green, the acidic solution has been neutralized.
4 Explain
Step 5
Explain how adding a base to an acidic solution affects the concentration of H3O+ ions.
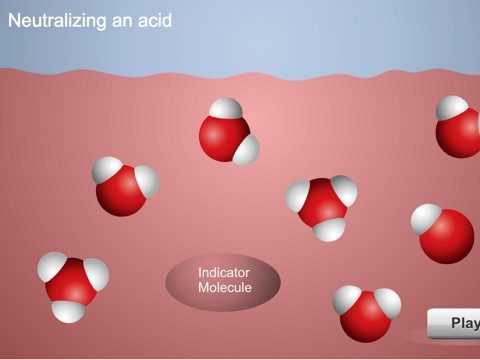
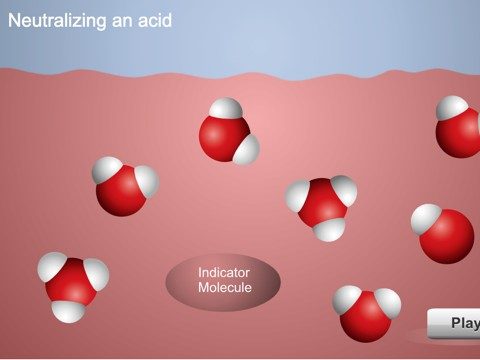
Project the animation Neutralizing an Acidic Solution.
Explain to students that adding drops of citric acid to the indicator solution increased the concentration H3O+ ions. When you add a base to this acidic solution, the base accepts protons from the water molecules creating OH− ions. The H3O+ ions and indicator molecule transfer protons to the OH− ions. When enough base is added so that the concentration of H3O+ and OH− ions becomes equal, the solution is neutralized.
5 Explore
Step 6
Have students compare how many more drops of a base it takes to neutralize a more concentrated acidic solution.
Question to Investigate
How many more drops of sodium carbonate solution will it take to neutralize a more concentrated citric acid solution?
Materials for Each Group
- Citric acid
- Citric acid solution
- Sodium carbonate solution
- Universal indicator solution
- 2 flat toothpicks
- 3 droppers
- Spot plate

Procedure
Neutralize a citric acid solution
- Use a flat toothpick to add two scoops of citric acid to your citric acid solution to make it even more acidic. Gently swirl until the citric acid dissolves.
- Add universal indicator solution to a clean well in the spot plate.
- Add 3 drops of the more concentrated citric acid solution to the indicator and stir with a clean toothpick.
Ask students:
- Do you think it will take more, less, or the same amount of sodium carbonate solution to neutralize this more concentrated citric acid solution?
It will take more drops of the base to neutralize the more concentrated citric acid solution. - Thinking about the animation, why will you need more drops of sodium carbonate solution?
Since the solution is more acidic, there are more H3O+ ions. So, it takes more molecules of the base to accept the extra protons and move toward neutral.
Procedure
Neutralize a more concentrated citric acid solution
- Add single drops of sodium carbonate solution to the same well in which you added the acid. Be sure to count the drops you use and stir with a toothpick after adding each drop. Record this number in the chart.
6 Extend
Step 7
Have students neutralize two basic solutions to determine which is most concentrated.
Materials for each group
- Universal indicator solution
- Citric acid solution
- Solution A
- Solution B
- At least 6 toothpicks
- Spot plate
- 3 droppers
Teacher Preparation
- Make two mystery solutions using different amounts of sodium carbonate.
- Label two cups Solution A and Solution B for each group.
- Make a class set of solutions A and B.
- Solution A: 50 mL of water and 5 toothpicks of sodium carbonate
- Solution B: 50 mL of water and 10 toothpicks of sodium carbonate
- Place about 5 mL of each solution in their labeled cups.
Ask students:
- Solutions A and B are both basic solutions made with sodium carbonate and water. One of these solutions has more sodium carbonate than the other. How can you figure out which solution is more concentrated?
Students should describe a procedure very similar to the one they used to neutralize the two citric acid solutions. They should suggest that they neutralize each sodium carbonate solution with drops of citric acid and count how many drops it takes to neutralize each solution. When the color of the solution is close to the color of the control, the solution is neutralized. - How will you know which solution is the most concentrated?
The solution that requires the greatest number of drops of citric acid to become neutral is the most basic.
Discuss what students will do:
- For best results, have students place 2 drops of Solution A in one well filled with indicator solution and 2 drops of Solution B in another well filled with indicator solution.
- Then they should add single drops of citric acid solution, stir, and compare the color to the color of the control.
- Students should keep track of the number of drops of citric acid it takes to neutralize each solution.
Procedure
- Add universal indicator solution to three wells in a clean spot plate.
- Leave the first well alone so that it can be used as a control. Add 2 drops of Solution A to the second well.
- Add 2 drops of Solution B to the third well.
Which solution is the most concentrated? | ||
Solution | Number of drops of solution added to the indicator | Number of drops of citric acid solution needed to neutralize the sodium carbonate solution |
Solution A | 2 drops |
|
Solution B | 2 drops |
|
- Neutralize Solution A and record the number of drops used in the chart.
- Neutralize Solution B and record the number of drops used in the chart.
Ask students:
- Which solution is the most concentrated? How do you know?
Students should discover that it takes more drops of citric acid to neutralize Solution B. Therefore, Solution B must be more concentrated than Solution A. - Antacids are medicines people take when the acid in their stomach is causing them discomfort. One advertisement says that the medicine provides relief for acid indigestion and sour stomach. What type of chemical do you think is in the medicine?
Bases neutralize acids, so the chemical is probably a base.
7 Extra Extend
Step 8
Place an Alka-Seltzer® tablet in indicator solution and have students interpret what the color changes say about the pH of the solution.
Explain that Alka-Seltzer® contains powdered acids and a base. The acids are citric acid, which tastes a little sour, and acetylsalicylic acid, which is aspirin. The base is baking soda, which is also known by its chemical name sodium bicarbonate.
Tell students that they will observe an Alka-Seltzer tablet in a universal indicator solution. Then they will use what they know about universal indicator and its color changes to describe whether the solution is acidic or basic as the substances in the tablet react.
Question to Investigate
How does the pH of the solution change during a chemical reaction between the ingredients in an Alka-Seltzer tablet in water?
Materials for Each Group
- Universal indicator solution in cup
- Water
- Alka-Seltzer tablet
- Graduated cylinder
- Snack-sized zip-closing plastic bag
Procedure
- Add 20 mL of universal indicator solution to a snack-sized zip-closing plastic bag.
- Seal the bag.
Note: So that students do not handle the Alka-Seltzer, which is a medicine, you should place an Alka-Seltzer tablet in each group’s bag.

Procedure for the Teacher
- Add an Alka-Seltzer tablet to each group’s bag by opening the corner of the bag just enough so that the tablet can fit through.
- Remove as much air as possible and drop the Alka-Seltzer tablet through the small opening.
- Seal the bag and hand it to one of the students. Instruct this student to shake the bag and pass it around so that each group member has an opportunity to hold the bag.
Expected Results
As soon as the Alka-Seltzer tablet is placed in the bag, the color of the indicator solution changes to red. Bubbles appear in the solution and the bag inflates. The solution also becomes cold. Over time the solution becomes orange, yellow, and finally returns to green.
Step 9
Discuss student observations.
As the colors are changing and the bags are inflating, ask students:
- What do the color changes tell you about the pH of the solution at the beginning, middle, and end of the chemical reaction?
Beginning: The solution is acidic.
Middle: The solution is becoming less acidic.
End: The solution is neutralized.
Students should conclude that the acid and base ingredients in the tablet neutralized one another.
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Downloads
For Students
- Lesson 6.9 Student Activity Sheet PDF | DOCX | Google Doc
For Teachers
- Lesson 6.9 Lesson Plan PDF | DOCX | Google Doc
- Lesson 6.9 Activity Sheet Answers PDF | DOCX | Google Doc
Resources for the entire Chapter 6
- Chapter 6 Student Reading PDF | DOCX | Google Doc
- Chapter 6 Test Bank PDF | DOCX | Google Doc
Interactive Lesson Modules
- Lesson 6.9 Online Assignments Google Form
Have Questions? Visit Help Center