Density: Sink and Float for Solids
Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts
- The density of an object determines whether it will float or sink in another substance.
- An object will float if it is less dense than the liquid it is placed in.
- An object will sink if it is more dense than the liquid it is placed in.
Summary
Students will investigate a wax candle and a piece of clay to understand why the candle floats and the clay sinks even though the candle is heavier than the piece of clay. Students will discover that it is not the weight of the object, but its density compared to the density of water, that determines whether an object will sink or float in water.
Objective
Students will be able to determine whether an object will sink or float by comparing its density to the density of water.

Safety
Be sure you and the students wear properly fitting goggles.
Materials for Each Group
- 2 tea light candles in their metal containers
- Clay
- Water in cup
- Small balance
- Tape
- Dropper
Notes About the Materials
A simple balance is required for the demonstration. One of the least expensive is Delta Education, Stackable Balance (21-inch) Product # 020-0452. Students can use the smaller version of the same balance, Delta Education, Stackable Balance (12-inch), Product # 023-0724.
You will need tea light candles for the demonstration and for each student group. Look for candles in which the wax completely fills the metal container.
Download All Lesson Resources
Get the entire lesson plan and Student Activity Sheet for "Lesson 3.4 - Density: Sink and Float for Solids."
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Instructions
1 Engage
Step 1
Do a demonstration to show that the wax is heavier than the clay but that the wax floats and the clay sinks.
Materials for the demonstration
- 1 tea light candle
- Clay
- Clear plastic container
- Water
- Large balance
Teacher preparation
- Use a small enough piece of clay so that you are sure that the candle weighs more than the clay.
- Pour water into a clear plastic container (or large cup) until it is about ½-full.

- Place a piece of clay that weighs less than a tea light candle on one end of a balance.
- Remove the candle from its metal container and place the candle on the other end of the balance.
- Ask students which is heavier, the clay or the candle. Ask them to predict which will sink and which will float. Then, place the clay and candle in a clear container of water.
Expected results
Even though the candle weighs more than the clay, the candle floats and the clay sinks.
Step 2
Discuss students’ ideas about why the heavier candle floats and the lighter clay sinks.
Ask students:
- Why do you think the bigger, heavier candle floats and the smaller, lighter clay sinks?
Students may say that the clay is more dense than wax. Tell students that the density of the objects is important, but that in sinking and floating, the density of the water matters, too. In fact, by comparing the density of an object to the density of water, you can predict whether an object will sink or float. One way to compare them is to weigh equal volumes of each.
2 Evaluate
Give each student an activity sheet.
- Lesson 3.4 Student Activity Sheet PDF | DOCX | Google Doc
- Lesson 3.4 Activity Sheet Answers PDF | DOCX | Google Doc
Download the student activity sheet, and distribute one per student.
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms and Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
3 Explore
Step 3
Have students compare the density of water, wax, and clay.
Question to investigate
Why does a heavier candle float and a lighter piece of clay sink?
Materials for each group
- 2 tea light candles in their metal containers
- Clay
- Water in cup
- Small balance
- Tape
- Dropper
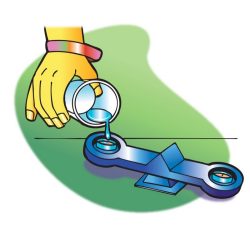
Procedure
Compare the density of wax and water
- Roll two pieces of tape and stick them to the center of the pan at each end of the balance.
- Attach each tea light candle to the tape so that each candle is in the center of the pan.
- Use the wick to pull one candle out of its container.
- Carefully pour water into the empty metal container until it fills the container to the same level as the candle in the other container. You may use a dropper to add the last bit of water and prevent spilling. The goal is to compare the mass of equal volumes of wax and water.
Expected results
The water has a greater mass than an equal volume of wax. So, the density of water must be greater than the density of wax.
Ask students:
- Which weighs more, wax or an equal volume of water?
Water weighs more than an equal volume of wax. - Which is more dense, wax or water?
Water is more dense.
If students have trouble understanding this relationship between the mass and density of equal volumes, have them think about the demonstration from Chapter 3, Lesson 1 with the aluminum and copper cubes. Both had the same volume, but the copper cube weighed more. Because the copper had more mass, it also had a greater density.
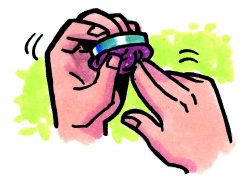
Compare the density of clay and water
- Make sure you have one piece of tape in the center of each pan on the balance.
- Fill one container with clay and place it on the tape so that it is in the center of the pan.
- Place an empty container on the tape at the opposite end of the balance.
- Slowly and carefully add water to the empty container until it is full.
Expected results
The clay has a greater mass than an equal volume of water. So, the density of clay is greater than the density of water.
Ask students:
- Which weighs more, the clay or an equal volume of water?
The clay weighs more than an equal volume of water. - Which is more dense, clay or water?
Clay is more dense. - Knowing the density of an object can help you predict if it will sink or float in water.
- If an object is more dense than water, would you expect it to sink or float?
Objects that are more dense than water sink.
- If an object is less dense than water, would you expect it to sink or float?
Objects that are less dense than water float.
- If an object is more dense than water, would you expect it to sink or float?
4 Explain
Step 4
Compare the density of wax, water, and clay on the molecular level.
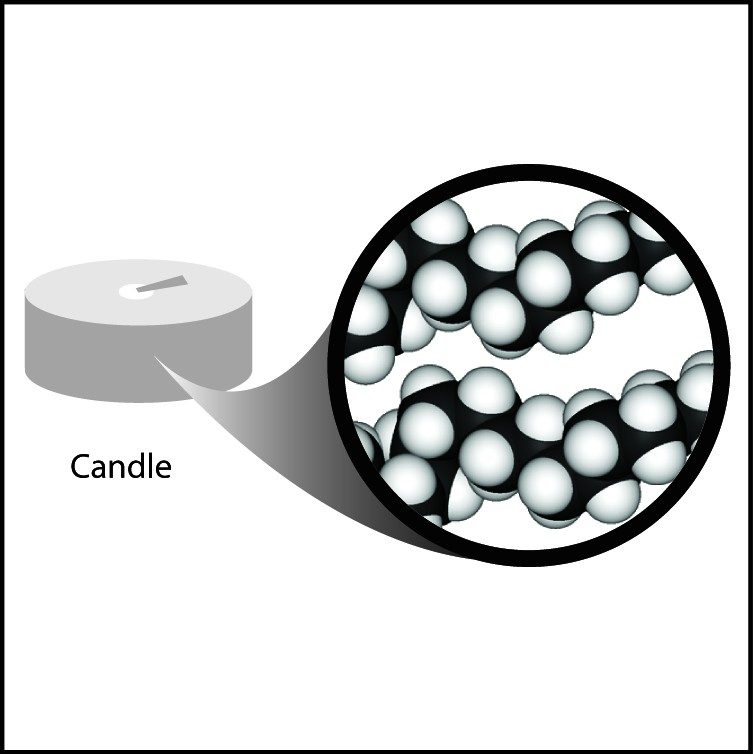
Project the image Wax.
Wax is made of carbon and hydrogen atoms connected together in long chains. These long chains are tangled, intertwined, and packed together to make the wax.
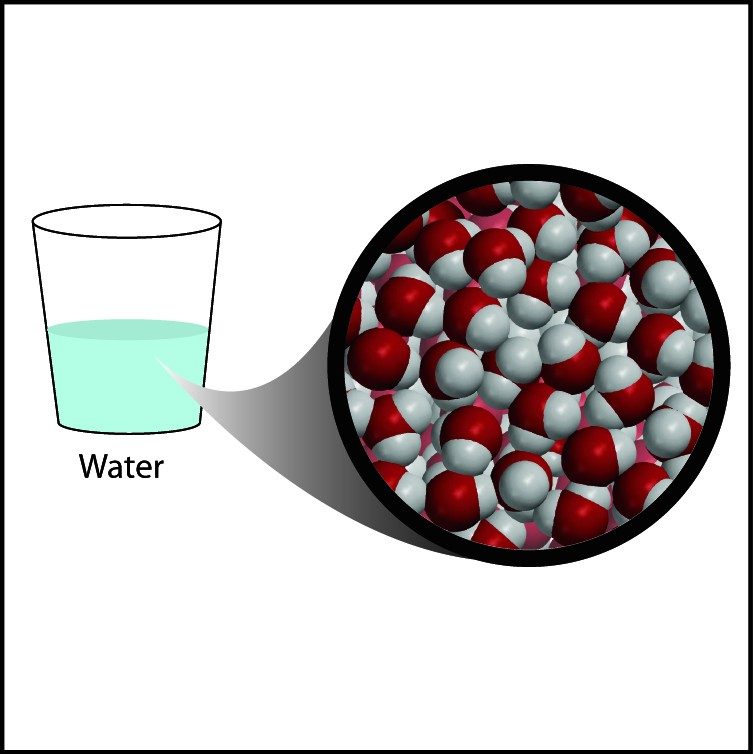
Project the image Water.
Even though they both have lots of hydrogen atoms, water is more dense than wax because the oxygen in water is heavier and smaller than the carbon in the wax. Also, the long chains of the wax do not pack as efficiently as the small water molecules.
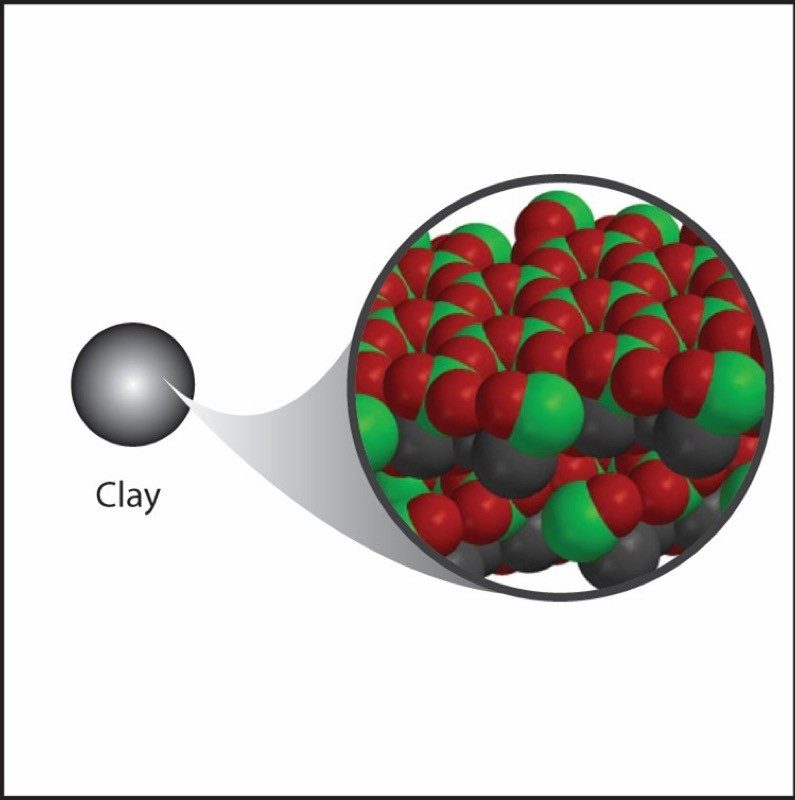
Project the image Clay.
Clay has oxygen atoms like water, but it also has heavier atoms like silicon and aluminum. The oxygen atoms are bonded to the silicon and aluminum to make molecules with a lot of mass. These are packed closely together, which makes the clay more dense than water.
5 Extend
Step 5
Have students explain, in terms of density, why a very heavy object like a big log floats and why a very light object like a tiny grain of sand sinks.
Ask students:
- A giant log can float on a lake, while a tiny grain of sand sinks to the bottom. Explain why a heavy object like the log floats while a very light grain of sand sinks.
Students should recognize that a log will float because wood is less dense than water. If you could weigh a large amount of water that has the same volume as the log, the log will weigh less than the water. Therefore, the log floats. A grain of sand will sink because sand is more dense than water. If you could weigh a small amount of water that has the same volume as the grain of sand, the sand will weigh more than the water. Therefore, the sand sinks.
Students should realize that if an object weighs more than an equal volume of water, it is more dense and will sink, and if it weighs less than an equal volume of water, it is less dense and will float.
- Remember that the density of water is about 1 g/cm3. Predict whether the following objects will sink or float.
Will these objects sink or float? | ||
|---|---|---|
Object | Density (g/cm3) | Sink or Float |
Cork | 0.2–0.3 |
|
Anchor | 7.8 |
|
Spruce wood oar | 0.4 |
|
Apple | 0.9 |
|
| Orange | 0.84 | |
| Orange without peel | 1.16 | |
Ask students:
- If a peach has a volume of 130 cm3 and sinks in water, what can you say about its mass?
Its mass must be more than 130 grams. - If a banana has a mass of 150 grams and floats in water, what can you say about its volume?
Its volume must be more than 150 cm3.
Note: Students may wonder why boats made out of dense material like steel can be made to float. This is a good question and there are several ways of answering it. A key to understanding this phenomenon is that the density of the material and the density of an object made of that material are not necessarily the same. If a solid ball or cube of steel is placed in water, it sinks.
But if that same steel is pounded and flattened thin and formed into a big bowl-like shape, the overall volume of the bowl is much greater than the volume of the steel cube. The mass of the steel is the same but the big increase in volume makes the density of the bowl less than the density of water so the bowl floats. This is the same reason why a steel ship is able to float. The material is shaped in such a way so that the density of the ship is less than the density of water.
Read more about sinking and floating in Teacher Background.
- Lesson 3.4 Teacher Background PDF
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Downloads
- Lesson 3.4 Student Activity Sheet PDF | DOCX | Google Doc
For Teachers
- Lesson 3.4 Lesson Plan PDF | DOCX | Google Doc
- Lesson 3.4 Activity Sheet Answers PDF | DOCX | Google Doc
- Lesson 3.4 Teacher Background PDF
Resources for the entire Chapter 3
- Chapter 3 Student Reading PDF | DOCX | Google Doc
- Chapter 3 Test Bank PDF | DOCX | Google Doc
Interactive Lesson Modules
- Lesson 3.4 Online Assignments Google Form
Have Questions? Visit Help Center