Chemical Change
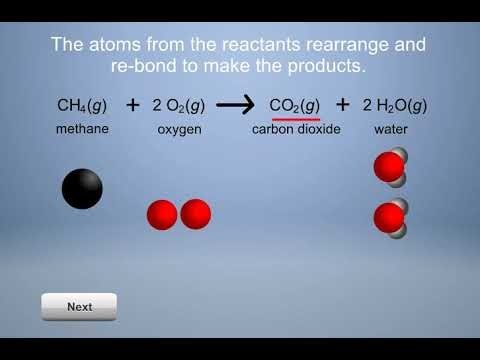
Students explore the concept that chemical reactions involve the breaking of bonds between atoms in the reactants, and the rearrangement and rebonding of these atoms to make the products. Students investigate reactions which produce a gas, form a precipitate, and cause a color change. Students also explore endothermic and exothermic reactions and do an engineering activity to design a device using an exothermic reaction.
Lessons:
- 6.1: What is a Chemical Reaction?
- 6.2: Controlling the Amount of Products in a Chemical Reaction
- 6.3: Forming a Precipitate
- 6.4: Temperature and Rate of a Chemical Reaction
- 6.5: A Catalyst and the Rate of Reaction
- 6.6: Using Chemical Change to Identify an Unknown
- 6.7: Energy Changes in Chemical Reactions
- 6.8: pH and Color Change
- 6.9: Neutralizing Acids and Bases
- 6.10: Carbon Dioxide Can Make a Solution Acidic
- 6.11: Chemical Reactions & Engineering Design
- 6.12: Natural Resources & Synthetic Materials
Downloads
Chapter 6
Student Reading
A summary of the investigations and science content from all lessons in the chapter
Teacher Background
At any point while using a lesson, you could familiarize yourself with the following content background.
Reading Materials
- Lesson 6.1 Teacher Background (PDF):
- Combustion of Methane
- Lesson 6.7 Teacher Background (PDF):
- Exothermic and Endothermic Chemical Reactions