Lesson 6.5: A Catalyst and the Rate of Reaction
Accompanying Lesson Plan: Lesson 6.5: A Catalyst and the Rate of Reaction
Video
“Elephant’s Toothpaste”
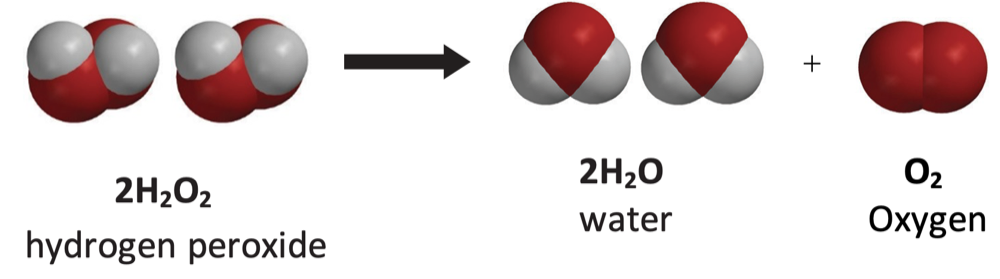
- Hydrogen peroxide decomposes on its own into water and oxygen gas.
- This process is sped up by a catalyst.
- In this reaction, the catalyst is potassium permanganate, and the bubbles are full of oxygen gas.
Video
“Genie in a Bottle”
- Hydrogen peroxide decomposes on its own into water and oxygen gas.
- This process is sped up by a catalyst.
- In this reaction, the catalyst is potassium permanganate.
- The reaction produces a lot of heat so the substance shooting out of the bottle is water vapor that is condensing in the cooler air.
- Oxygen gas is invisible but is mixed in with the water vapor.