Lesson 6.7: Energy Changes in Chemical Reactions
Accompanying Lesson Plan: Lesson 6.7: Energy Changes in Chemical Reactions
Video
Thermite Reaction
- Iron oxide, aluminum, and a catalyst are placed in a flowerpot.
- The reaction is extremely exothermic resulting in molten iron and aluminum dripping into sand below the flowerpot.
The demonstration shown in this video is very dangerous. Do not attempt to perform this demonstration.
Video used with permission from Chemical Education Exchange (ChemEd X)
Video
Nitrogen Triiodide Reaction
- The nitrogen triiodide is so unstable that the slightest touch will cause it to decompose in an exothermic reaction.
The demonstration shown in this video is very dangerous. Do not attempt to perform this demonstration.
Video used with permission from Chemical Education Exchange (ChemEd X)
Video
White Phosphorous Reaction
- White phosphorous dissolved in a solvent is dripped onto a piece of paper.
- When the solvent evaporates, the phosphorous combines with oxygen in an exothermic reaction.
The demonstration shown in this video is very dangerous. Do not attempt to perform this demonstration.
Video used with permission from Chemical Education Exchange (ChemEd X)
Interactive
Methane Combustion – Energy
- The combustion of methane is an example of an exothermic reaction. More energy is released when the bonds in the products are formed than is used to break the bonds of the reactants. This is shown by larger energy arrows coming out of the products and smaller energy arrows going into the reactants.
Interactive
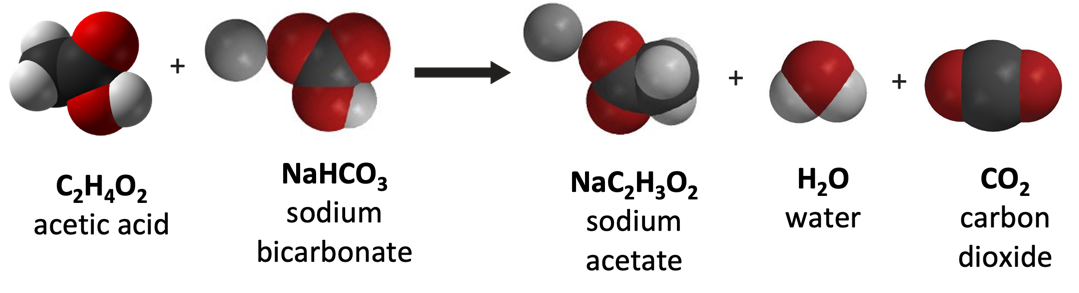
Endothermic Reaction
- Energy is used to break the bonds of the reactants.
- Energy is released when the bonds of the products are formed.
- Since more energy is absorbed when the bonds are broken than is released when new bonds are formed, the reaction is endothermic, and the temperature goes down.
Interactive
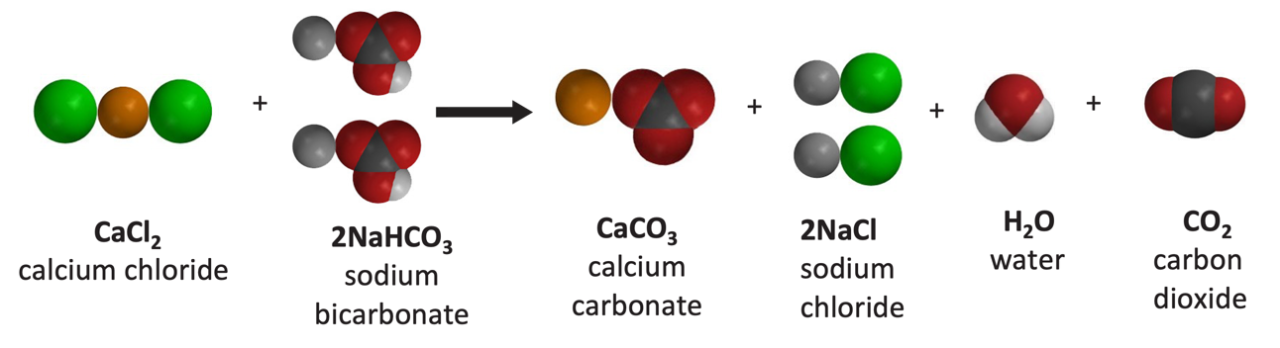
Exothermic Reaction
- Energy is used to break the bonds of the reactants.
- Energy is released when the bonds of the products are formed.
- Since more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants, the reaction is exothermic, and the temperature goes up.