Lesson 6.8: pH and Color Change
Accompanying Lesson Plan: Lesson 6.8: pH and Color Change
Interactive
Proton Transfer in Water
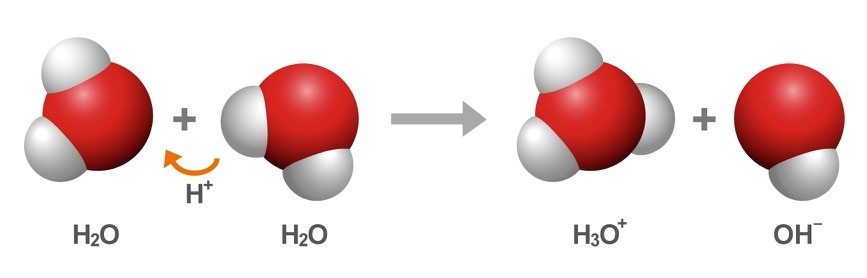
- The hydrogen atom in the water molecule has one proton and an electron that it shares with the oxygen atom. A proton from a hydrogen atom can transfer to another water molecule, creating the ions H3O+ and OH-.
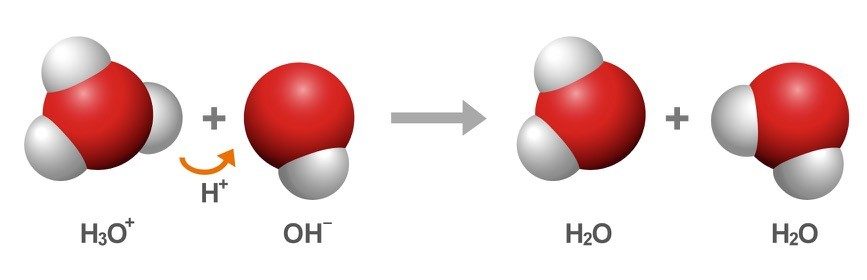
- A proton from the H3O+ ion can be transferred to the OH-, creating water molecules again.
Image
Water Molecules Trade Protons
- The hydrogen atom in the water molecule has one proton and an electron that it shares with the oxygen atom. A proton from a hydrogen atom can transfer to another water molecule, creating the ions H3O+ and OH-
- A proton from the H3O+ ion can be transferred to the OH-, creating water molecules again.
Interactive
Acids Donate Protons
- An acid added to water donates protons to the water molecules. This increases the concentration of H3O+ ions. The H3O+ ions donate protons to the indicator molecules which results in a color change.
Interactive
Bases Accept Protons
- A base added to water accepts protons from the water molecules. This increases the concentration of OH- ions. The H3O+ ions and the indicator molecules donate protons to the OH- ions. This decreases the concentration of H3O+ ions and results in a color change.