Controlling the Amount of Products in a Chemical Reaction
Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts
- Changing the amount of reactants affects the amount of products produced in a chemical reaction.
- In a chemical reaction, only the atoms present in the reactants can end up in the products.
- Mass is conserved in a chemical reaction.
Summary
Students will analyze the chemical equation for the reaction between vinegar (acetic acid solution) and baking soda (sodium bicarbonate). They will make the connection between the written chemical equation, the molecular model, and the real substances in the reaction. Students will see that the gas produced in the actual reaction is also written in the products of the equation. Students will also change the amount of one or more reactants and see how the change affects the amount of products.
Objective
Students will be able to explain that for a chemical reaction to take place, the bonds between atoms in the reactants are broken, the atoms rearrange, and new bonds between the atoms are formed to make the products. Students will be able to count the number of atoms on the reactant side and on the product side of a chemical equation. They will also be able to explain that the equal number of atoms on each side of the equation shows that mass is conserved during a chemical reaction.
Students will also be able to explain, on the molecular level, why changing the amount of one or more reactants changes the amount of products. They will also be able to explain why simply adding more and more of one reactant will eventually not produce additional products.

Safety
Be sure you and the students wear properly fitting goggles. Use vinegar in a well-ventilated room. Have students wash hands after the activity.
Materials for the Demonstrations
- Vinegar
- Baking soda
- Water
- Alka-Seltzer
- Detergent solution
- Graduated cylinder (50 mL)
- Graduated cylinder (100 mL)
- Measuring spoon (½ teaspoon)
- 1 clear plastic cup
- Small cup
- Dropper
- Plastic waste container
Materials for Each Group
- Vinegar in a cup
- Baking soda in a cup
- Detergent solution in a cup
- Dropper
- Graduated cylinder (50 mL)
- Measuring spoons (⅛, ¼, and ½ teaspoon)
- Plastic waste container
Download All Lesson 6.2 Resources
Get the entire lesson plan and Student Activity Sheet for "Lesson 6.2 - Controlling the Amount of Products in a Chemical Reaction."
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Instructions
1 Engage
Step 1
Have students look at the chemical equation for the vinegar and baking soda reaction as you discuss the reactants.
Remind students that in the last lesson, they learned that in a chemical reaction, certain atoms in the reactant molecules unbond from one another and then rearrange and rebond in different ways to form the products. Students saw that the same type and number of atoms were in the reactants as were in the products. Let students know that although the reaction in this lesson looks more complicated, these same principles still apply.
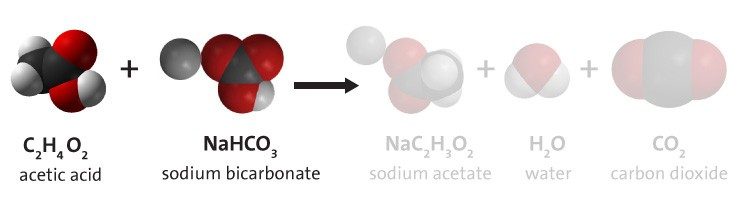
Project the image Reactants.
Show students the chemical equation for the reaction between vinegar and baking soda.
Ask students about vinegar:
- Acetic acid mixed with water is vinegar. Usually, vinegar is a solution of about 5% acetic acid and 95% water. When a reactant is in solution, the water is usually not listed as a reactant. Which atoms make up a molecule of acetic acid (vinegar)?
Carbon, hydrogen, and oxygen (C, H, and O). - What do the little numbers below and to the right of each letter mean?
These are the number of that particular atom in the acetic acid molecule. There are two carbon atoms, four hydrogen atoms, and two oxygen atoms in an acetic acid molecule. - Do you think every acetic acid molecule has this formula?
Yes. The chemical formula for a substance is unique to that substance and defines what it is.
Ask students about baking soda:
- Sodium bicarbonate is baking soda. What atoms is sodium bicarbonate made of?
Sodium, hydrogen, carbon, and oxygen (Na, H, C, and O). - How many of each type of atom are there in the compound sodium bicarbonate?
There are one sodium atom, one hydrogen atom, one carbon atom, and three oxygen atoms in every unit of sodium bicarbonate.
Step 2
As a demonstration, combine vinegar and baking soda to show students the chemical reaction described in the equation.
Materials for the Demonstration
- Vinegar
- Graduated cylinder (50 mL)
- Baking soda
- Clear plastic cup

Procedure
- Use a graduated cylinder to measure 10 mL of vinegar.
- Place about ½ teaspoon of baking soda in a clear plastic cup.
- While students watch, pour the vinegar into the baking soda.
Expected Results
Bubbles will form and rise in the cup.
Ask students:
- A liquid and a solid were combined and you saw bubbling, which is made from gas. Do you think a chemical reaction occurred? Why?
A chemical reaction occurred because a different substance was produced when the reactants combined.
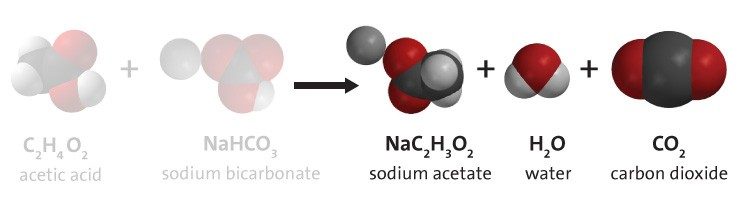
Project the image Products.
Point out the products in the chemical reaction.
Ask students:
- Look at the chemical equation. What is the gas produced in the chemical reaction between vinegar and baking soda?
Carbon dioxide - What else is produced in this chemical reaction?
When vinegar and baking soda react, atoms rearrange to form sodium acetate (the salty and sour flavor in salt-and-vinegar-flavored potato chips), water, and carbon dioxide.
Continue to project the chemical equation as you and students count the number of atoms on both the reactant side and product side of the equation.
Step 3
Review the concept that mass is conserved in a chemical reaction.
Help students count the atoms in the reactants and in the products of the vinegar-baking soda reaction. Make sure students see that every type of atom on the left side of the equation is also on the right. Also be sure that they see that there is an equal number of each type on both sides of the equation.
Guide students as you answer the following questions together:
- Is every type of atom on the left side of the equation also on the right side of the equation? Yes. Why?
Atoms from the reactants rearrange to form the products. Atoms are not created or destroyed in a chemical reaction. - How many of each type of atom is on the reactant side of the equation?
3 carbon atoms, 5 hydrogen atoms, 5 oxygen atoms, and 1 sodium atom. - How many of each type of atom is on the product side of the equation?
3 carbon atoms, 5 hydrogen atoms, 5 oxygen atoms, and 1 sodium atom.
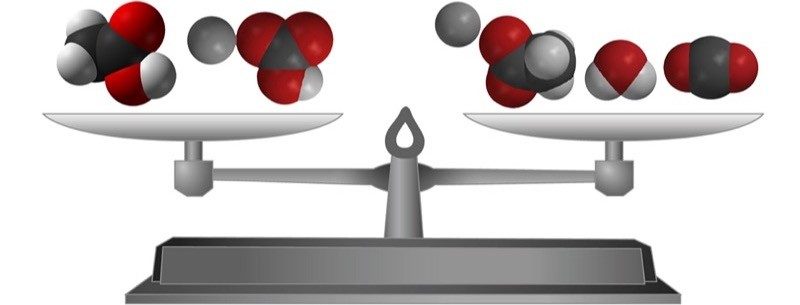
Project the image Mass is Conserved.
Point out that the type and number of atoms in the reactants and in the products are exactly the same. This is an important concept in chemistry: In a chemical reaction, all the atoms in the reactants end up in the products. When an equation of a chemical reaction is written, it is “balanced” to show this. A balanced chemical equation shows that no atoms are destroyed and no new atoms are created in the chemical reaction. Explain to students that another way of saying that no atoms are created or destroyed in a chemical reaction is that mass is conserved.
2 Explore
Step 4
As a demonstration, combine vinegar, detergent, and baking soda in a graduated cylinder so that foam rises and spills over the top.
Teacher Preparation for the Demonstration and for Each Group
- Make a detergent solution by adding 1 teaspoon of liquid dish detergent to 2 tablespoons of water. Divide this detergent solution equally into one small cup for each group.
- Place about 1 tablespoon of vinegar in a small cup for each group.
- Place about 2 teaspoons of baking soda in a small cup for each group.
Materials for the Demonstration
- Vinegar
- Baking soda
- Detergent solution
- Dropper
- Graduated cylinder (50 mL)
- Measuring spoon (½ teaspoon)
- Plastic waste container
- Small cup
Procedure
- Use a graduated cylinder to measure 10 mL of vinegar.
- Pour the vinegar in a small cup and add 1 drop of detergent. Swirl gently to mix.
- Add ½ teaspoon of baking soda to the empty graduated cylinder.
- Place the graduated cylinder in a plastic waste container.
- Pour the vinegar and detergent from the cup into the graduated cylinder. Have students observe the level of foam in the graduated cylinder.
- Rinse the graduated cylinder over the waste container.
Expected Results
White foam will rise in the graduated cylinder and overflow.
Step 5
Discuss how to change the amount of foam produced so that it rises to the top of the cylinder without overflowing.
Ask students:
- What could you change to create a foam that rises as close as possible to the top of the cylinder without overflowing?
Students might mention variables such as:- The amount of vinegar, detergent, or baking soda.
- The order in which the substances are added to the graduated cylinder.
Explain that the amount of detergent should not be varied in this activity because it is used as an indicator to help measure the amount of gas produced in the reaction. Also, the baking soda should be added to the cylinder first. The vinegar is poured in afterwards to cause better mixing of reactants.
Remind students that 10 mL of vinegar and ½ teaspoon of baking soda caused the foam to overflow. Students should consider these amounts as they plan how much of each reactant they will use as they start their trials.
Ask students:
- Can you add the baking soda first and then the vinegar on one trial and then switch it for the other trials? No. Why not?
Every test should be conducted the same way. For example, in the demonstration baking soda was placed in the graduated cylinder before the vinegar and detergent were added. This method mixes the baking soda and vinegar well. All new trials should be conducted this same way. - Should you rinse the graduated cylinder each time? Yes. Why?
Any products or leftover reactants that remain in the graduated cylinder may affect the next reaction. It is best to rinse the cylinder after each trial. - How will you remember the amounts you used in each trial?
Students should realize the necessity of making and recording accurate measurements in the chart provided.
Give each student an activity sheet.
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms & Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually, depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
- Lesson 6.2 Student Activity Sheet PDF | DOCX | Google Doc (See Answers in all downloads)
Step 6
Have each group experiment with different amounts of vinegar and baking soda to get the foam to rise to the top of the graduated cylinder without overflowing.
Tell students that they should try to get the foam to stop as close as possible to the top of the cylinder without overflowing. You may choose to limit students to a maximum of three tries or let them experiment further if time and supplies allow.
Question to Investigate
How can you make just the right amount of foam that rises to the top of the graduated cylinder without overflowing?
Materials for Each Group
- Vinegar in a cup
- Baking soda in a cup
- Detergent solution in a cup
- Dropper
- Graduated cylinder (50 mL)
- Measuring spoons (⅛, ¼, and ½ teaspoon)
- Plastic waste container
Procedure
- Decide on how much vinegar and baking soda you will use and write these amounts in the chart on the activity sheet.
- Use a graduated cylinder to measure the amount of vinegar your group agreed on.
- Pour the vinegar in a small cup and add 1 drop of detergent. Swirl gently to mix.
- Add the amount of baking soda your group agreed on to the empty graduated cylinder.
- Place the graduated cylinder in a plastic waste container.

- Pour the vinegar and detergent from the cup into the graduated cylinder. Observe the level of foam in the graduated cylinder.
- Rinse the graduated cylinder over the waste container.
Expected Results
Using ⅛ teaspoon of baking soda, 5 mL of vinegar, and 1 drop of detergent will probably cause the foam to rise to the top of the cylinder without overflowing. Results may vary.
Have groups share their findings about the amounts of baking soda and vinegar that came closest to reaching the top of the cylinder. Did each group use similar amounts of baking soda and vinegar?
3 Evaluate
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
- Lesson 6.2 Student Activity Sheet PDF | DOCX | Google Doc
- Lesson 6.2 Student Activity Sheet Answers PDF | DOCX | Google Doc
4 Explain
Step 7
Discuss why adjusting the amount of reactants affects the amount of products.
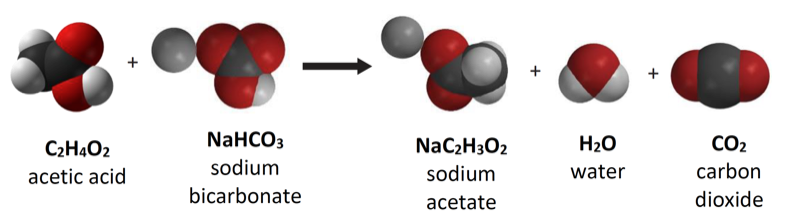
Project the image Controlling Amount of Products Formed.
Show students the chemical equation for the reaction between vinegar and baking soda.
Ask students:
- Why, on the molecular level, does changing the amount of baking soda or vinegar affect the amount of carbon dioxide gas produced?
Products are made from the reactants, so adding more reactants will produce more of the products.
The important point for students to realize is that atoms from both reactants are necessary to produce the products. Using less baking soda, for instance, produces less carbon dioxide gas because there are fewer atoms from the baking soda to produce the carbon dioxide. In general, using more of one or more reactants will result in more of one or more products. Using less of one or more reactants will result in less of one or more products. Let students know that this principle has limits.
Note: It is not necessary for students at the middle school level to know which particular atom in the reactants ended up in which product. It might seem strange, but sometimes a product can be made up of atoms from only one reactant. In the vinegar and baking soda reaction, the atoms in the CO2 only come from the sodium bicarbonate.
Ask students:
- What would you do if you wanted to make more carbon dioxide?
Add more vinegar and more baking soda. - Could you just keep adding more and more baking soda to the same amount of vinegar to get more carbon dioxide?
No. This might work for a while, as long as there is extra vinegar, but eventually there would be no atoms left of vinegar to react with the extra baking soda, so no more carbon dioxide would be produced.
5 Extend
Step 8
Do a demonstration using Alka-Seltzer or a similar effervescent tablet in water to show that citric acid reacts with sodium bicarbonate to produce carbon dioxide gas.
Tell students that an Alka-Seltzer tablet contains aspirin, sodium bicarbonate, and citric acid. Citric acid interacts with the sodium bicarbonate similar to the way the acetic acid in vinegar interacts with sodium bicarbonate.
Ask students to make a prediction:
- What will happen when an Alka-Seltzer tablet is placed in water with a drop of detergent solution?
Materials for the Demonstration
- Alka-Seltzer
- Water
- Graduated cylinder (100 mL)
- Detergent solution
- Dropper

Procedure
- Place 50 mL of water in a 100 mL graduated cylinder.
- Add 1 drop of detergent solution and swirl gently to mix.
- Drop half of an Alka-Seltzer tablet in the graduated cylinder.
- Place the graduated cylinder in a waste container.
Expected Results
White foam will rise in the graduated cylinder and overflow as the tablet becomes smaller and smaller.
Ask students:
- Do you think this is a chemical reaction? Yes. Why?
Because a gas was produced. This gas was not in one of the reactants, so it must have been produced during the chemical reaction. - Why do you think this reaction is similar to the reaction of vinegar and baking soda?
Citric acid and vinegar are both acids and so interact with sodium bicarbonate in a similar way to produce carbon dioxide gas.
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Downloads
For Students
- Lesson 6.2 Student Activity Sheet PDF | DOCX | Google Doc
For Teachers
- Lesson 6.2 Lesson Plan PDF | DOCX | Google Doc
- Lesson 6.2 Activity Sheet Answers PDF | DOCX | Google Doc
Resources for the entire Chapter 6
- Chapter 6 Student Reading PDF | DOCX | Google Doc
- Chapter 6 Test Bank PDF | DOCX | Google Doc
Interactive Lesson Modules
- Lesson 6.2 Online Assignments Google Form
Have Questions? Visit Help Center