Lesson 6.1: What is a Chemical Reaction?
Accompanying Lesson Plan: Lesson 6.1: What is a Chemical Reaction?
Image
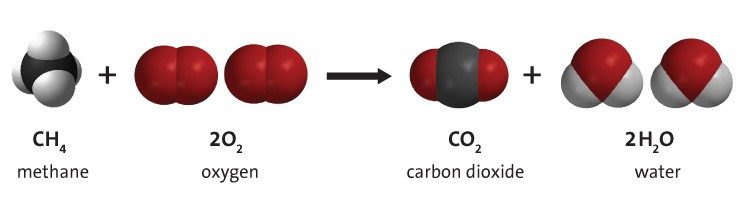
Methane and Oxygen React
- In the reaction, the bonds in the methane and oxygen molecule come apart, the atoms rearrange and then re-bond to form water and carbon dioxide.
- The little number written at the lower right after an atom (subscript) tells how many of that atom are in the molecule.
- The big number written in front of a molecule (coefficient) shows how many of that molecule there are.
- All the atoms in the products come from the atoms in the reactants.
Interactive
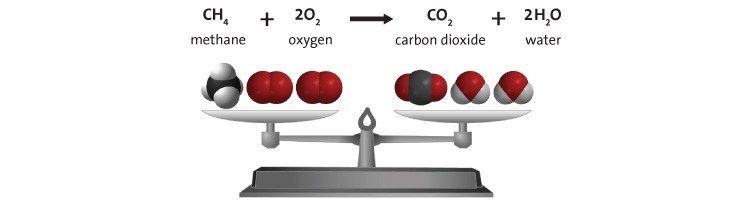
Combustion of Methane
- The reactants are on the left side of the equation and the products are on the right.
- In the reaction, the bonds in the methane and oxygen molecule come apart, the atoms rearrange and then re-bond to form water and carbon dioxide.