Lesson 5.9: Temperature Changes in Dissolving
Accompanying Lesson Plan: Lesson 5.9: Temperature Changes in Dissolving
Interactive
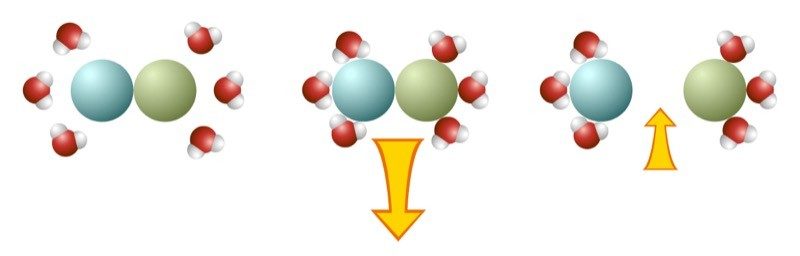
Breaking and Making Bonds
- When two atoms or molecules that are attracted to each other are together, it takes energy to break them apart.
- When two atoms or molecules that are attracted to each other are apart, energy is released when they form a bond.
- It takes energy to break bonds and energy is released when bonds are formed.
Interactive
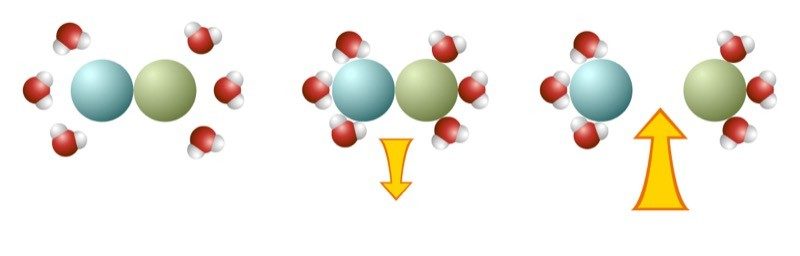
Energy and Dissolving
- When water molecules are attracted to and bond to the solute, energy is released.
- It takes energy when water molecules pull on the solute, causing it to separate.
Video
Hand Warmer
- The liquid in the hand warmer is a very concentrated solution of a salt called sodium acetate.
- The sodium and acetate ions are ready to bond with each other and with water molecules to form a crystal.
- When these ions and molecules attract and bond to each other, energy is released.
Video
Temperature Change – Alcohol in Water
- When alcohol dissolves in water, water molecules “bond” to alcohol molecules and separate them from other alcohol molecules.
- The energy released when alcohol and water molecules attract and bond is greater than the energy required to separate alcohol molecules from each other.
- Since the energy released is greater than the energy absorbed, the dissolving of alcohol in water is exothermic.