Lesson 5.3: Why Does Water Dissolve Salt?
Accompanying Lesson Plan: Lesson 5.3: Why Does Water Dissolve Salt?
Image
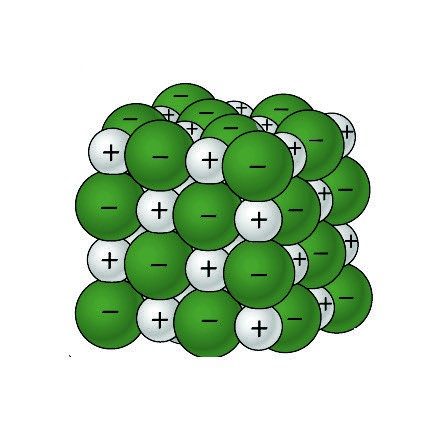
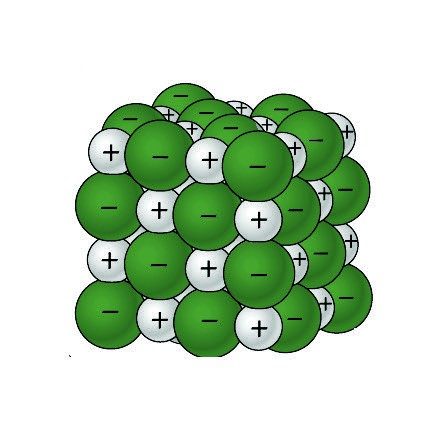
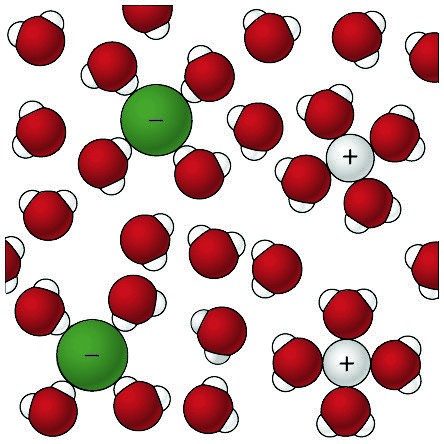
Sodium Chloride Dissolving in Water 1
- The positive areas of the water molecules surround the negative Chloride ions.
- The negative areas of the water molecules surround the positive sodium ions.
- As the attractions from the water molecules and their motion pulls the ions apart, the sodium chloride crystal dissolves.
Video
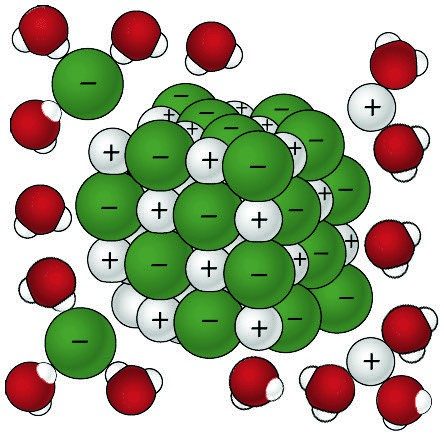
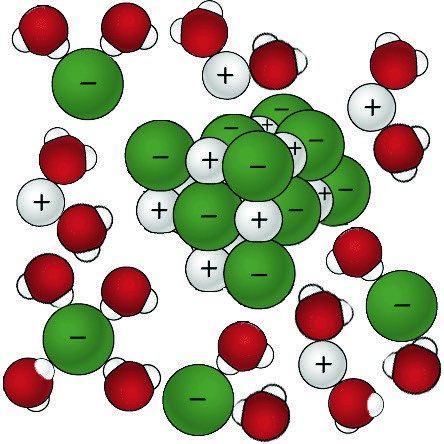
Sodium Chloride Dissolving in Water 2
- The positive areas of the water molecules surround the negative Chloride ions.
- The negative areas of the water molecules surround the positive sodium ions.
- As the attractions from the water molecules and their motion pulls the ions apart, the sodium chloride crystal dissolves.
Video used with permission from Roy Tasker, VisChem Project