Lesson 5.4: Why Does Water Dissolve Sugar?
Accompanying Lesson Plan: Lesson 5.4: Why Does Water Dissolve Sugar?
Image
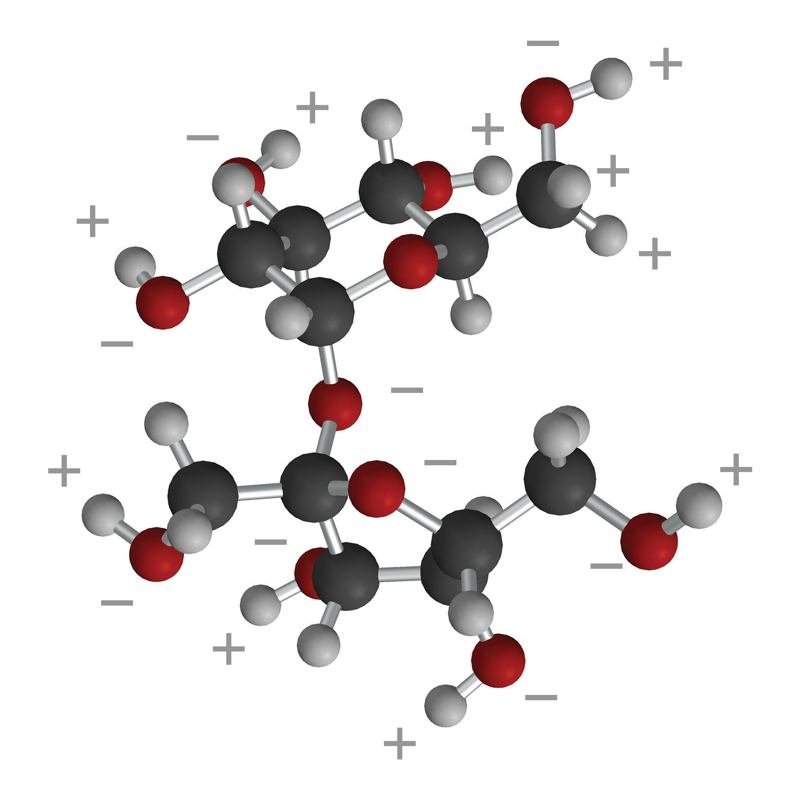
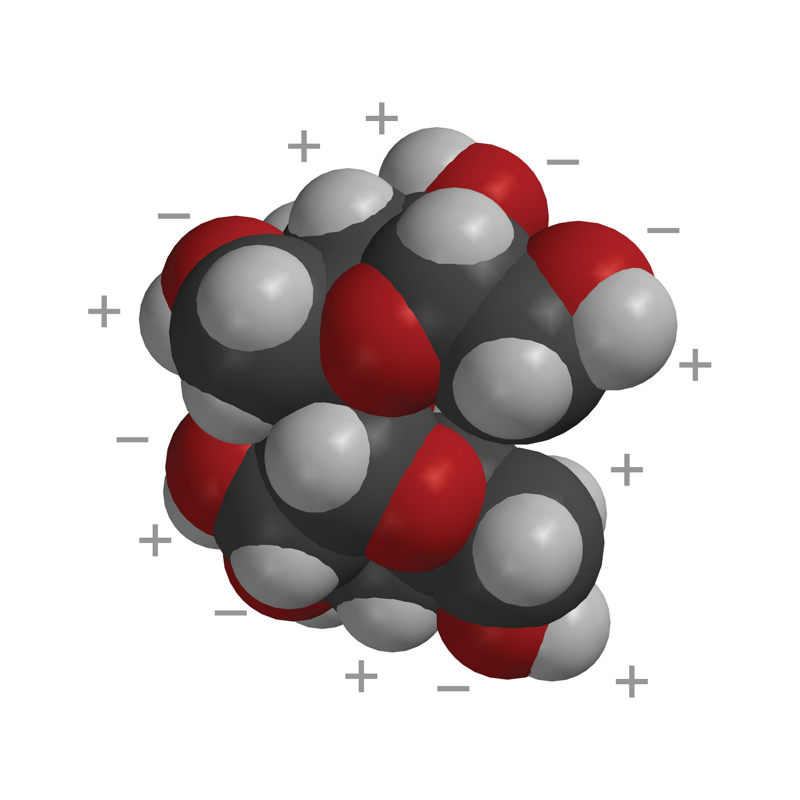
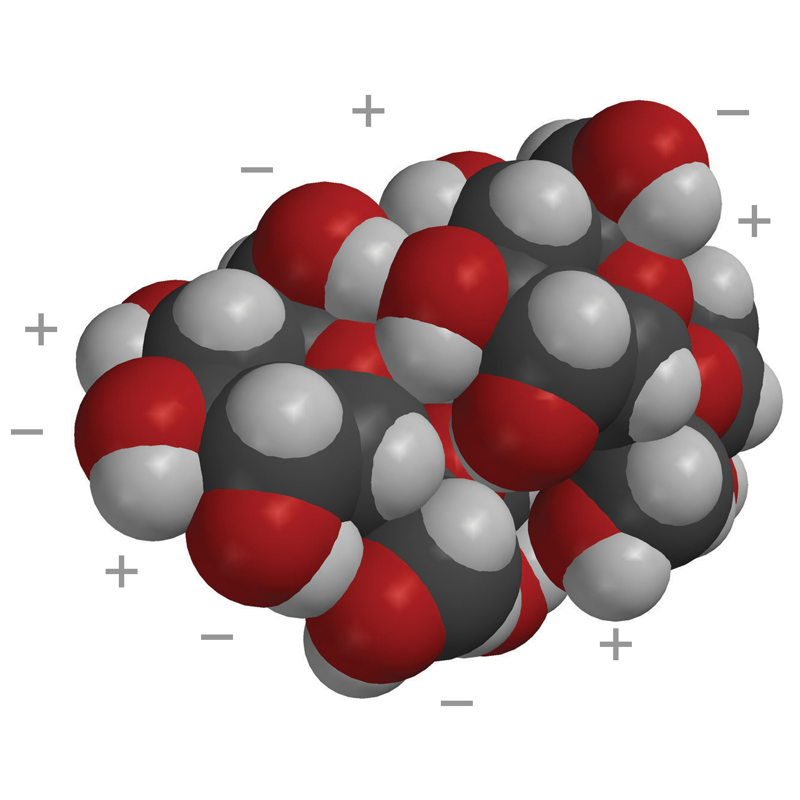
Sucrose 1
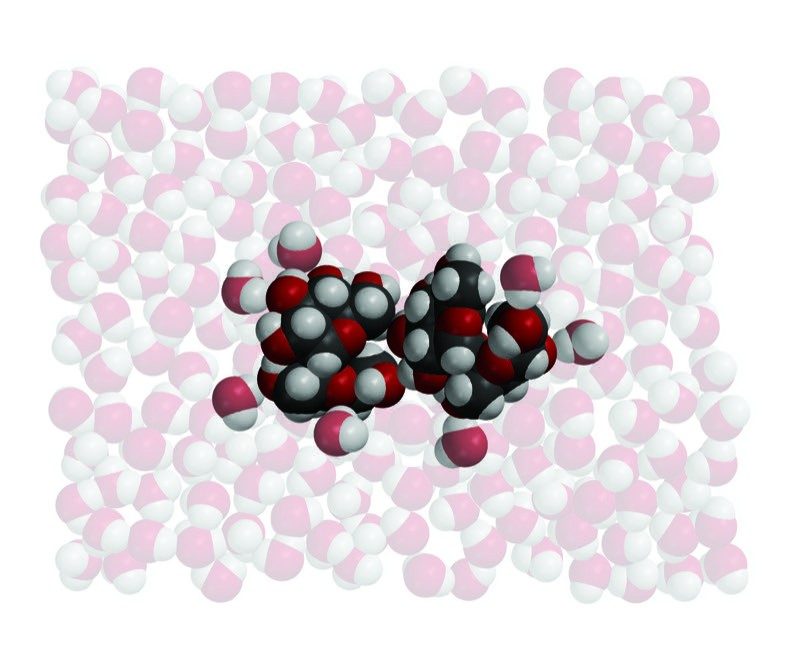
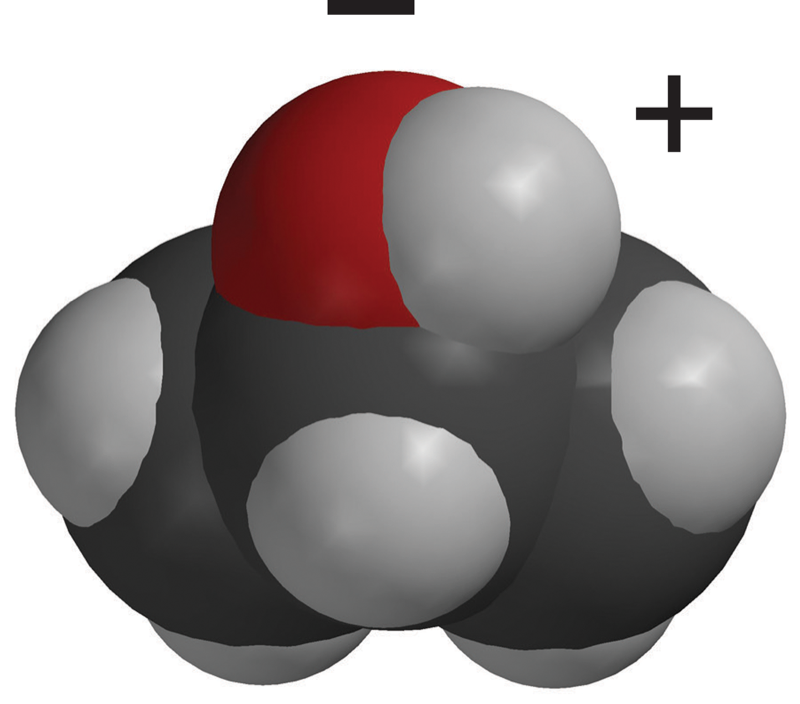
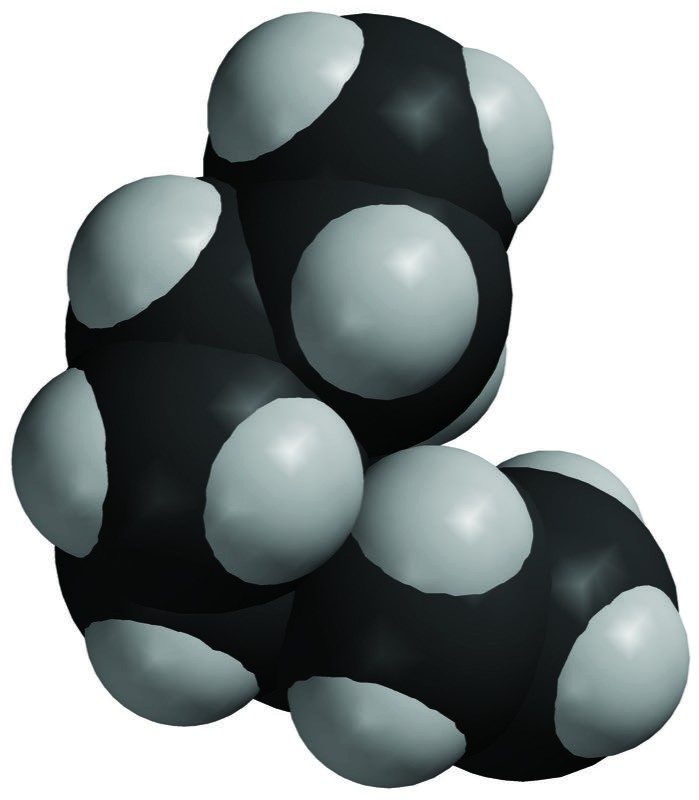
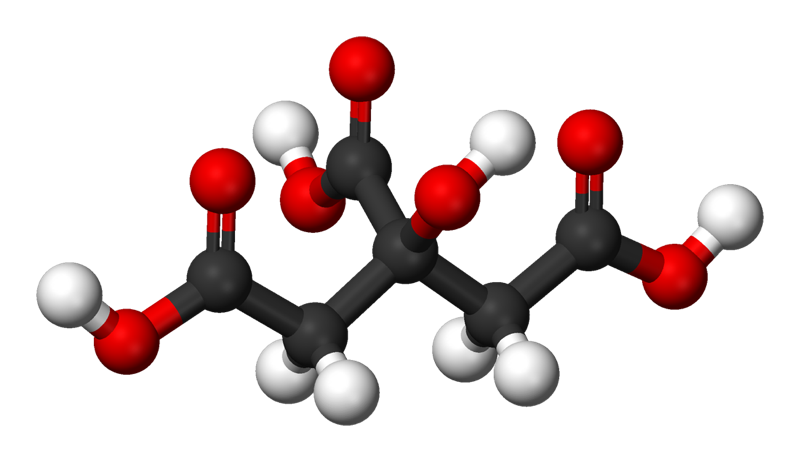
- The ball-and-stick and first space-filling model show that sucrose is a large molecule made up of carbon, oxygen, and hydrogen.
- Sucrose has many O–H bonds which are polar.
- These polar areas are shown with a + near the hydrogen atom and a − near the oxygen atom.
- The second space-filling model shows two sucrose molecules held together by their opposite polar areas.
- These molecules will separate from each other when sucrose dissolves.
Image
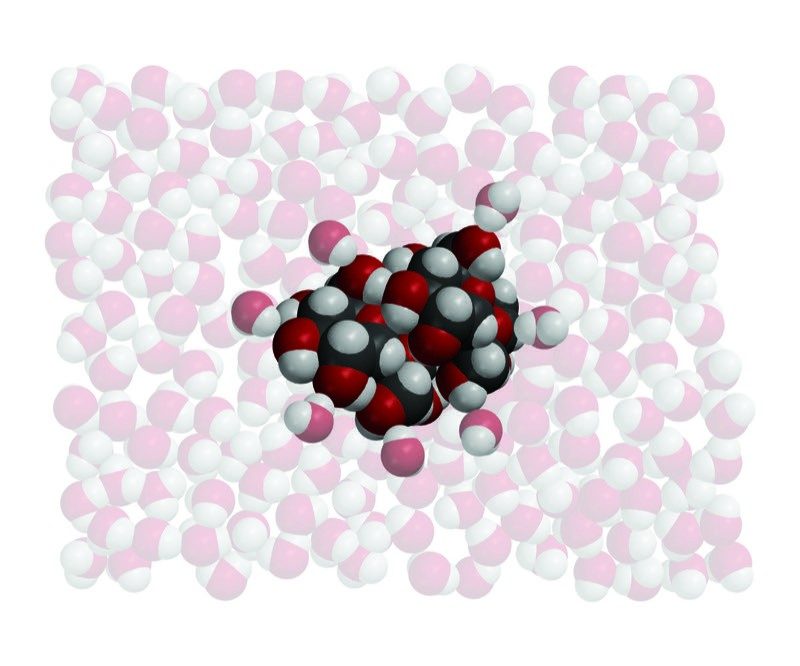
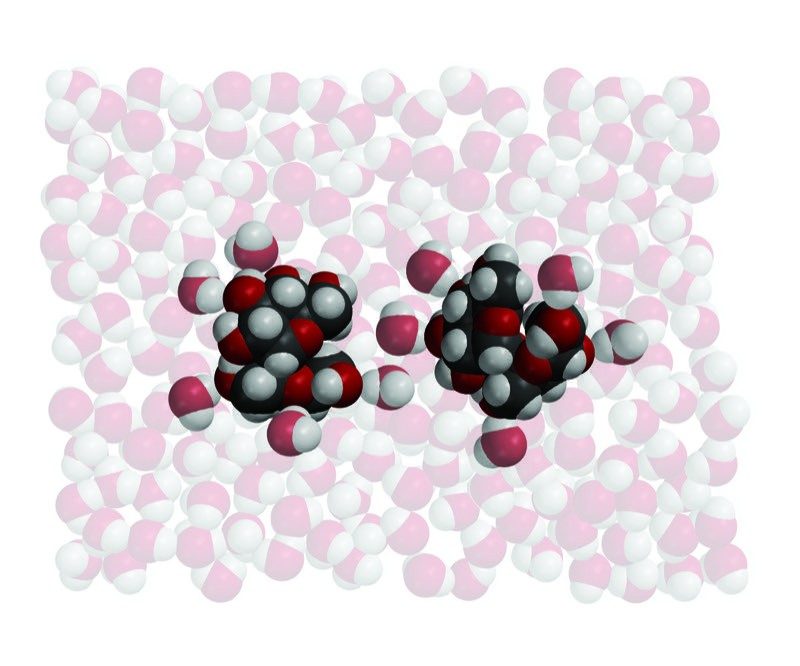
Water Dissolves Sucrose 1
- Water molecules arrange themselves around the sucrose molecules according to opposite polar areas.
- The attraction of the water molecules and their motion overcome the attraction between sucrose molecules.
- The sucrose molecules dissolve as they are separated from the other molecules and mix into the water.
Interactive
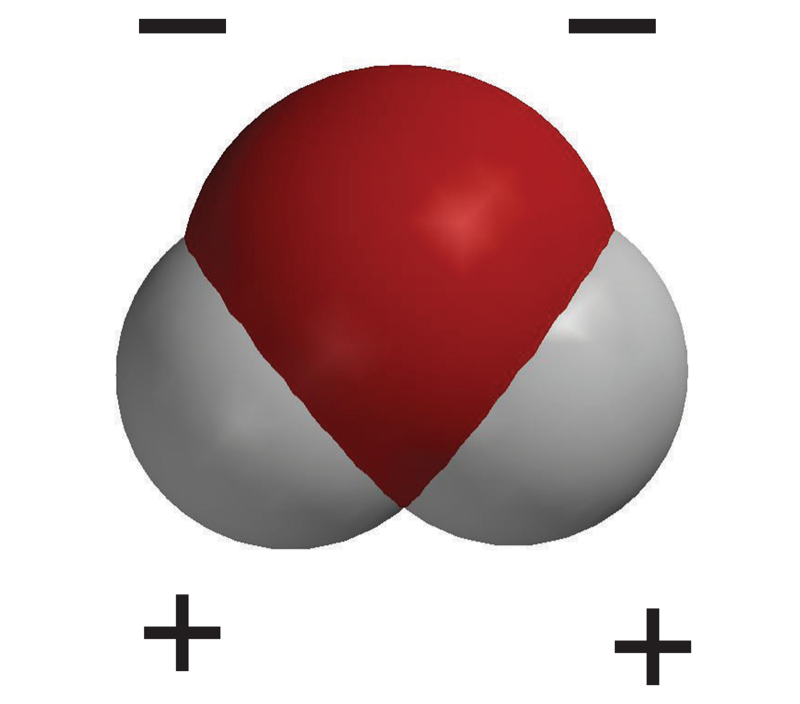
Sucrose
- The sucrose molecule has many oxygen-hydrogen (O–H) bonds which are polar.
- The charge-density model shows the positive areas near the hydrogen atom as blue and the negative area near the oxygen atom as red.
- The sugar molecules are attracted and held together in a crystal by these opposite polar areas.
Video
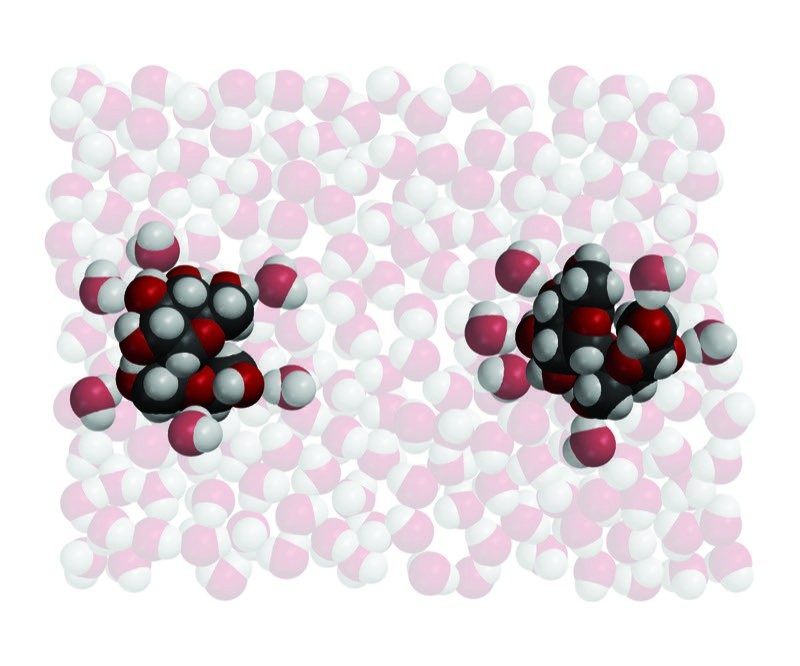
Water Dissolves Sucrose 2
- Water molecules arrange themselves around the sucrose molecules according to opposite polar areas.
- The attraction of the water molecules and their motion overcome the attraction between sucrose molecules.
- The sucrose molecules dissolve as they are separated from each other and mix into the water.