Lesson 5.5: Using Dissolving to Identify an Unknown
Accompanying Lesson Plan: Lesson 5.5: Using Dissolving to Identify an Unknown
Image
All Four Crystals
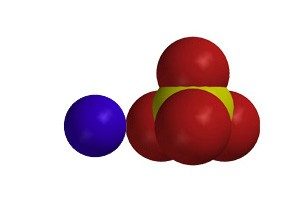
- Epsom salt is an ionic compound. There is a positive magnesium ion (Mg2+) and a negative sulfate ion (SO42−). Polar water interacts with these oppositely charged ions to dissolve the Epsom salt.
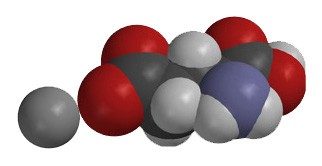
- MSG is made of a positive sodium ion (Na+) and a negative glutamate ion, which has the molecular formula (C5H8NO4−). Polar water interacts with these oppositely charged ions to dissolve the MSG.
- Salt—sodium chloride is an ionic compound. There is a positive sodium ion (Na+) and a negative chloride ion (Cl−). Polar water interacts with these oppositely charged ions to dissolve the salt.
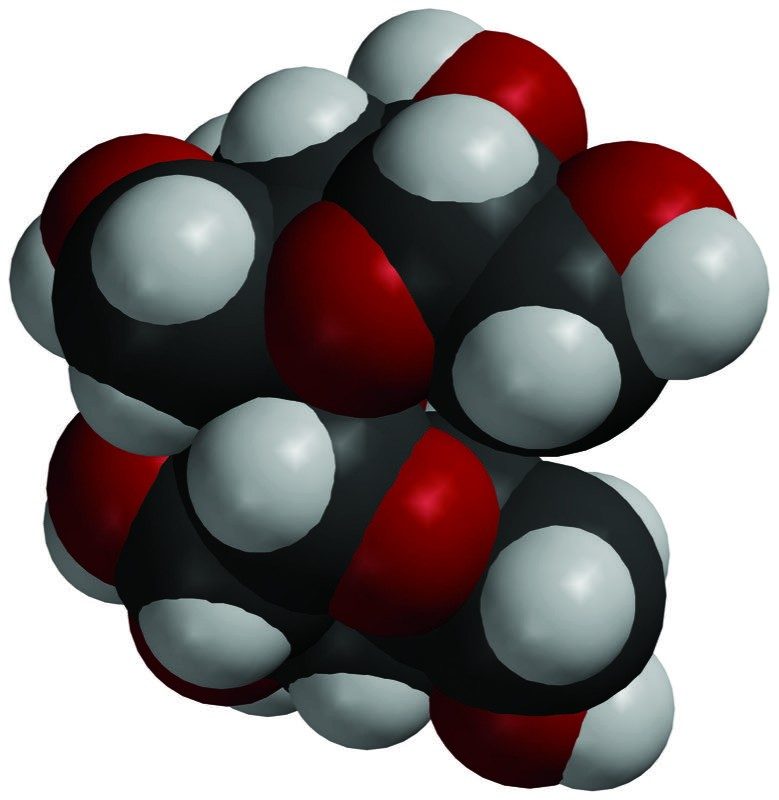
- Sucrose is not an ionic compound. Sucrose has many O–H bonds, which give it positive and negative polar areas. These areas attract other sucrose molecules and hold them together in a crystal. These polar areas interact with water and cause entire sucrose molecules to separate from one another and dissolve.