Lesson 1.5: Air, It's Really There
Accompanying Lesson Plan: Lesson 1.5: Air, It's Really There
Video
Air Has Mass, Basketball
- The deflated basketball has a mass of 576 grams.
- After adding air, the inflated basketball has a mass of 581 grams.
- Therefore, the mass of the air added was 5 grams.
Video
Air Has Mass, Can
- The can of compressed gas has a mass of 135 grams.
- After letting gas out of the can, it has a mass of 132 grams.
- Therefore, the gas that escaped had a mass of 3 grams.
Interactive
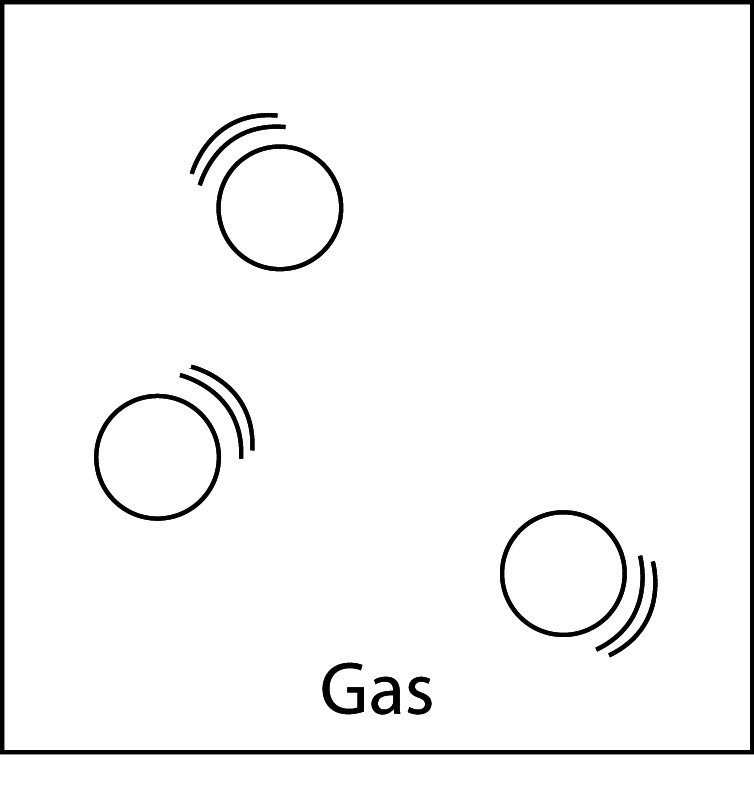
Particles of a Gas
- The particles (atoms or molecules) of a gas have very little attraction for one another.
- They barely interact with each other. If they collide, they usually just bounce off.
- The particles of a gas are much further apart than the particles in a liquid or a solid.
Interactive
Heating and Cooling Gas in a Bottle
- The red arrows in the animation represent outside air pushing down on the bubble film.
- When the bottle is heated, the molecules inside the bottle move faster, the faster moving molecules inside the bottle push against the bubble film, and this push from the inside air overcomes the push from the outside air, forming a bubble.
- When the bottle is cooled, the molecules inside the bottle move slower, the slower moving molecules inside the bottle push less hard against the bubble film, and the outside air overcomes the push from the inside air, forcing the bubble down.
Interactive
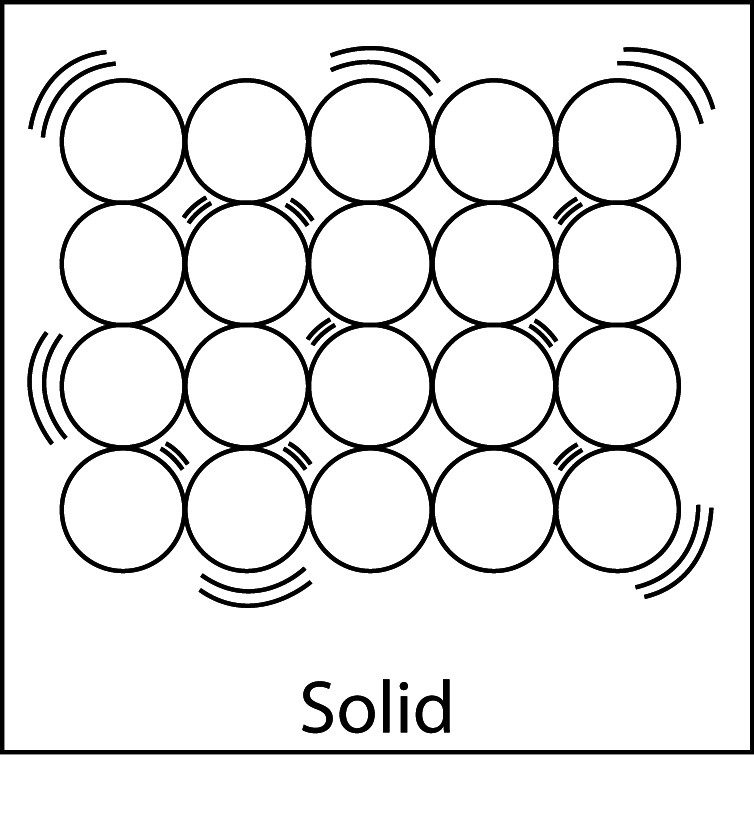
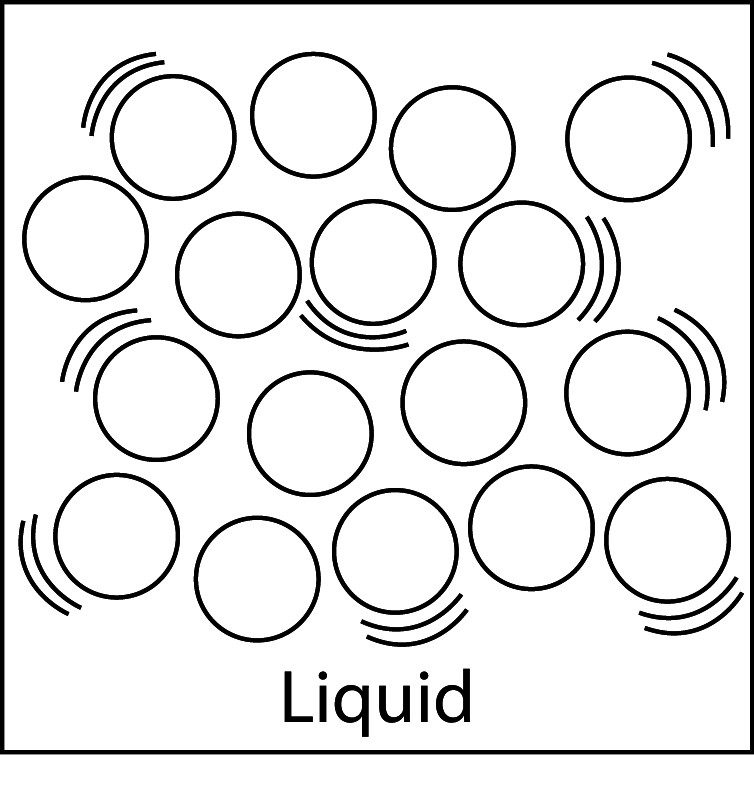
Comparing Solids, Liquids, and Gases
- The molecules shown are from three different substances that are all at room temperature.
- In the solid, molecules are strongly attracted to one another they vibrate but do not move past one another, and molecules stay in fixed positions because of their strong attractions for one another. A solid has a definite volume and a definite shape.
- In the liquid, molecules are attracted to one another, molecules vibrate but are also able to move past one another, a liquid has a definite volume but does not have a definite shape.
- In the gas, molecules are not attracted to each other much at all. The molecules in gas vibrate and are also able to move freely past each other. A gas does not have a definite shape of volume. Gas molecules will spread out evenly to fill any container.
Image
Solid, Liquid, and Gas
- In a solid, the particles have strong attractions, orderly arrangement, and are close together. Solids have a definite volume and shape.
- In a liquid, the attractions between particles are not as strong as in solids. They are randomly arranged and slightly further apart. Liquids have a definite volume, but not a definite shape.
- In a gas, the particles are arranged randomly, are very far apart, and the attractions between them are weak. Gases have no definite volume or shape.
Interactive
Heating Molecules of a Gas
- The molecules of a gas have very little attraction for one another and so barely interact with each other.
- When the molecules of a gas are heated, they move faster.
- The faster moving molecules inside the balloon push against the material the balloon is made out of.
- This push from the inside air overcomes the inward pull of the balloon, making the balloon get bigger.