Lesson 2.3: Changing State—Condensation
Accompanying Lesson Plan: Lesson 2.3: Changing State—Condensation
Video
Condensation Cup
- When a cup filled with cold water is exposed to air, the water in the air condenses onto the side of the cup.
- If the cup of cold water is not exposed to air, no condensation will form.
Interactive
Condensation
- The fast-moving molecules of water vapor transfer their energy to the side of the cup which is cooler. This causes the water vapor molecules to slow down. When they slow down enough, their attractions overcome their speed and they stay together as liquid water.
Note: The particles on the side of the cup may look like a solid, but in this case, they are meant to represent a drop of liquid.
Image
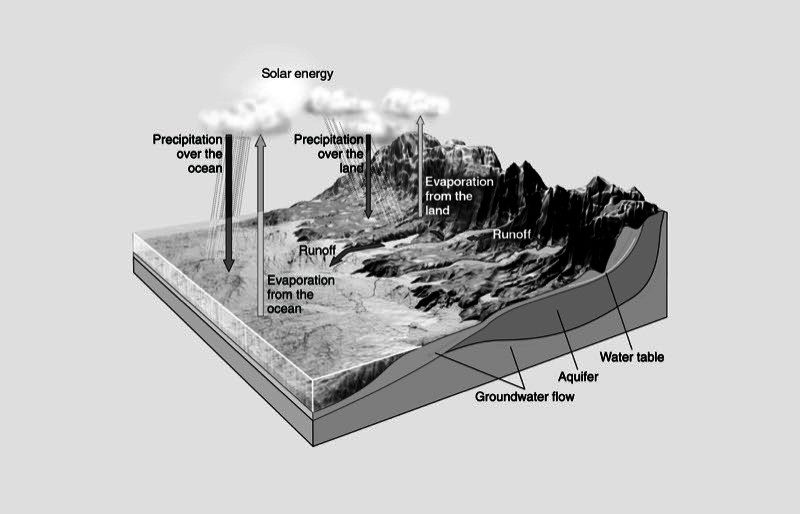
Water Cycle
- Energy from the sun speeds evaporation of water from the ocean and from water on the land.
- Cooler temperatures in the upper atmosphere cause water vapor to condense to tiny droplets which form clouds.
- When the clouds become saturated, it rains and the cycle continues.
Video
Evaporation and Condensation
- Water molecules break away the surface of the water (evaporate) and also rejoin the water (condense).
- In a closed jar or terrarium, the rate of evaporation and condensation would eventually become equal.
Video used with permission from Roy Tasker, VisChem Project