Lesson 2.2: Changing State—Evaporation
Accompanying Lesson Plan: Lesson 2.2: Changing State—Evaporation
Interactive
Evaporation
- Heating the water on the paper towel increases the motion of the water molecules.
- When the molecules have enough energy, they can move fast enough to break away from the attractions holding them to other molecules.
Image
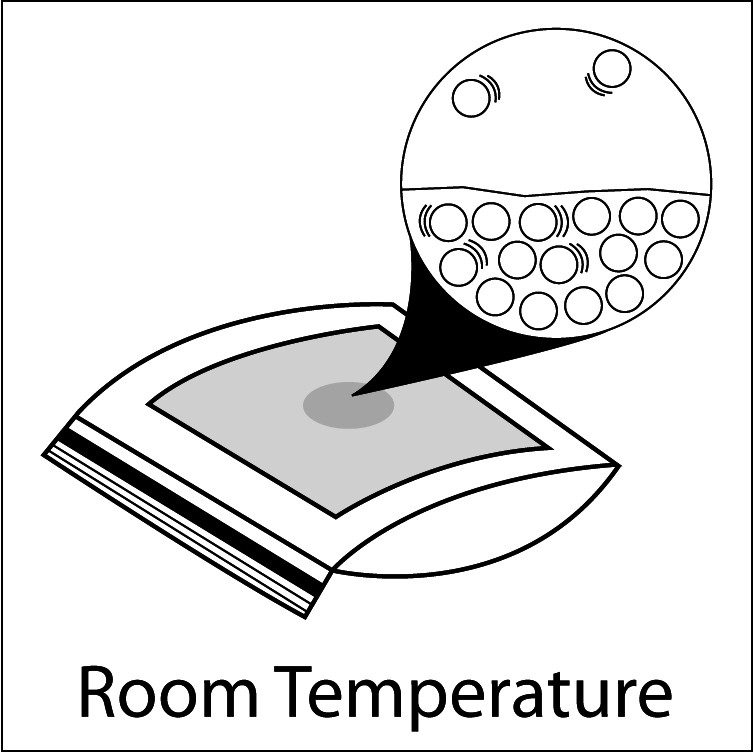
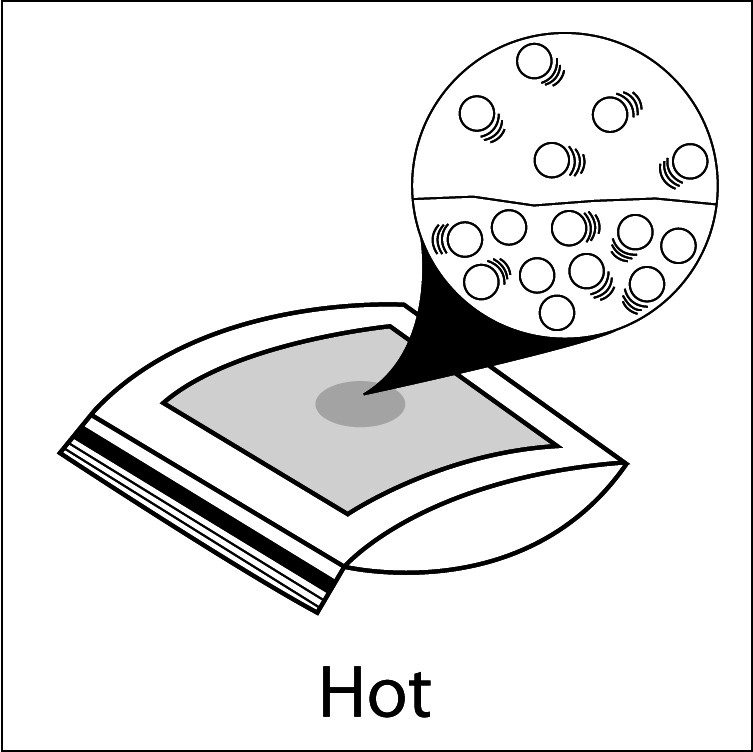
Heating and Evaporation
- Compare the number of motion lines and the spacing of the water molecules in the water on each paper towel. Heated water molecules have more energy and move faster than the room temperature water molecules. These faster moving molecules are able to overcome the attractions they have for other water molecules more easily and evaporate.
Video
Models of Water Molecules
- The ball-and-stick model shows that atoms are bonded at certain angles in the molecule.
- The space-filling model shows the region taken up by the electron clouds of the atoms in the molecule.
Video used with permission from Roy Tasker, VisChem Project
Video
Liquid Water
- The molecules in liquid water are attracted to one another but are able to slide past each other. If you look closely, you can see some molecules where the hydrogen atoms of one molecule are “bonded” to the oxygen of another.
Note: The water molecules are shown further apart than they would be so that molecules in the background can be seen.
Video used with permission from Roy Tasker, VisChem Project
Video
Water Vapor
- Molecules are very far apart. After evaporating, the molecules of water vapor has separated from the molecules in the liquid water.
- The molecule itself does not break apart into individual atoms.
Video used with permission from Roy Tasker, VisChem Project