Lesson 3.1: What is Density?
Accompanying Lesson Plan: Lesson 3.1: What is Density?
Image
Aluminum and Copper
- Copper atoms are larger than aluminum atoms.
- There are fewer copper atoms than aluminum atoms in the cubes.
- Copper atoms are much more massive than aluminum atoms.
- The extra mass makes copper more dense than aluminum.
Interactive
Density of a Cube
- Volume is a measure of the amount of space an object takes up. It is always in three dimensions. To find the volume of an objects like a cube or a box, measure the length × width × height. If measured in centimeters, the answer will be in cubic centimeters (cm³).
- Mass is measured in grams.
- The units for density will be g/cm³.
Image
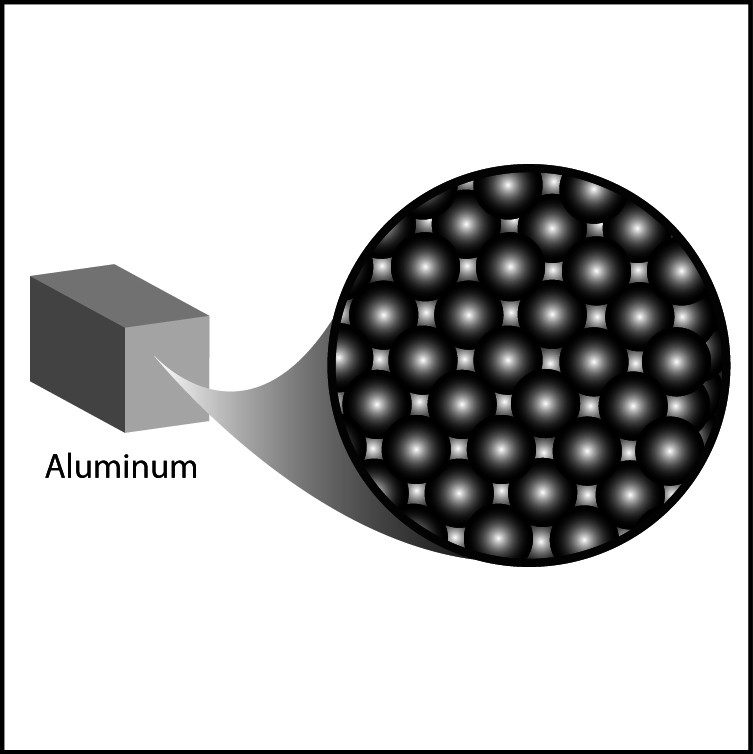
Metal
- Arrangement of atoms in different metals is similar.
- Individual atoms are packed very close together.
- Therefore, differences in density between metals are usually based on the size and mass of the atoms.
Image
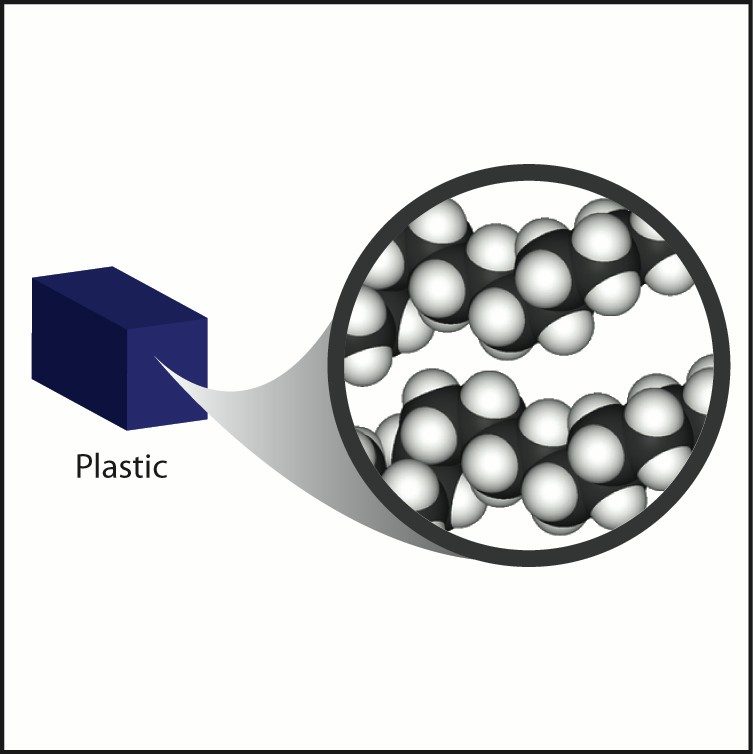
Plastic
- Most plastics are made from chains of carbon and hydrogen atoms connected together.
- Molecules in plastics can have: different length chains, twisted, tangled, or layered chains, different atoms or molecules connected to the C–H chain.
- If the different atoms are heavy atoms, the plastic tends to be more dense, if they are light atoms, the plastic tends to be less dense.
Image
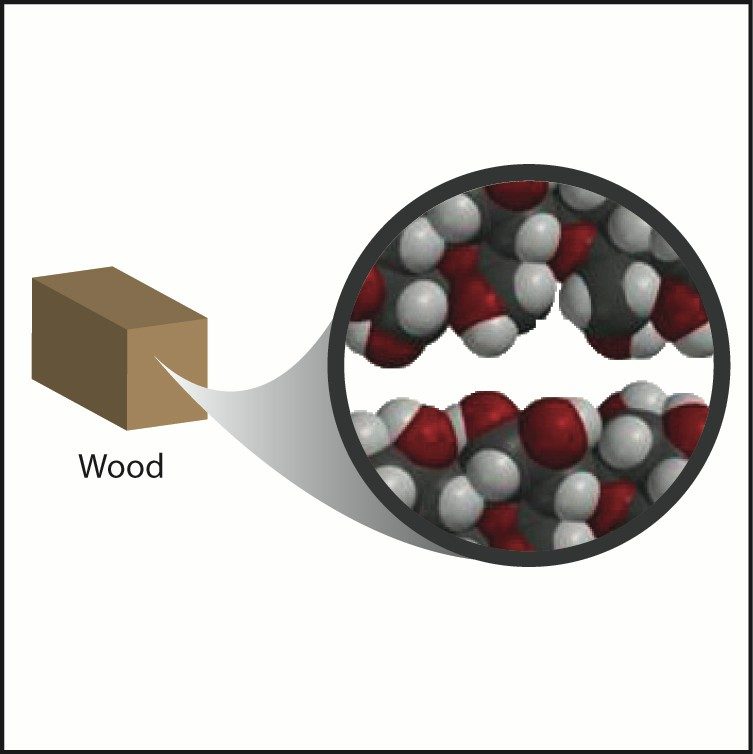
Wood
- Wood is made from a long molecule called cellulose.
- Cellulose is made from glucose molecules connected together in long chains.
- Glucose is made from carbon, hydrogen, and oxygen atoms.
- The structure of wood is made of many cellulose molecules stacked together. Plastic and wood can have similar densities because they are made from similar atoms and are both composed of long chains. The main difference is how closely packed the chains are to one another.