Lesson 5.8: Can Gases Dissolve in Water?
Accompanying Lesson Plan: Lesson 5.8: Can Gases Dissolve in Water?
Image
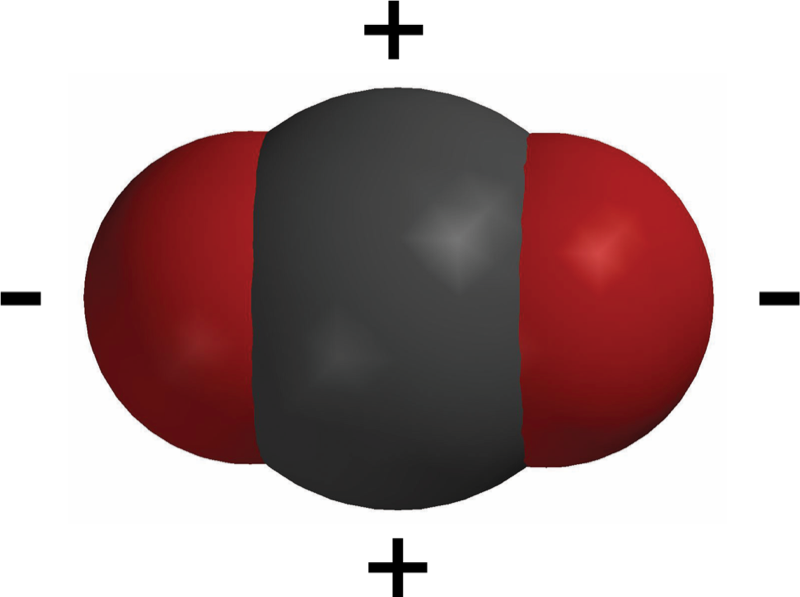
Carbon Dioxide (CO2) Molecule
- Carbon dioxide is made up of one carbon atom covalently bonded to two oxygen atoms.
- Although electrons are shared, they are not shared equally between the carbon and the oxygen atoms.
- The oxygen atom has a stronger pull on electrons than the carbon atom.
- This makes the oxygen ends of the molecule slightly negative and the carbon atom slightly positive.
Video
Mentos and Diet Coke Demo
- Although the candies seem smooth, they have microscopic rough spots.
- The carbon dioxide molecules in the soda attach to these rough spots or nucleation points on the surface of the candies.
- The carbon dioxide builds up into bubbles and the increased pressure pushes the gas and the soda out of the bottle.